Chemistry Gas Laws Test Answers . Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. The number of particles in the gas increases as the volume increases. Why is a gas easier to compress than a. You will most likely see pv = nrt as one. Identify the choice that best completes the statement or answers the question. Boyle’s law, charles’s law, and avogadro’s law, as well as. Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: The following practice problems are to master to topics on the ideal gas laws:
from www.studocu.com
You will most likely see pv = nrt as one. Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. The following practice problems are to master to topics on the ideal gas laws: Why is a gas easier to compress than a. Boyle’s law, charles’s law, and avogadro’s law, as well as. Identify the choice that best completes the statement or answers the question. The number of particles in the gas increases as the volume increases.
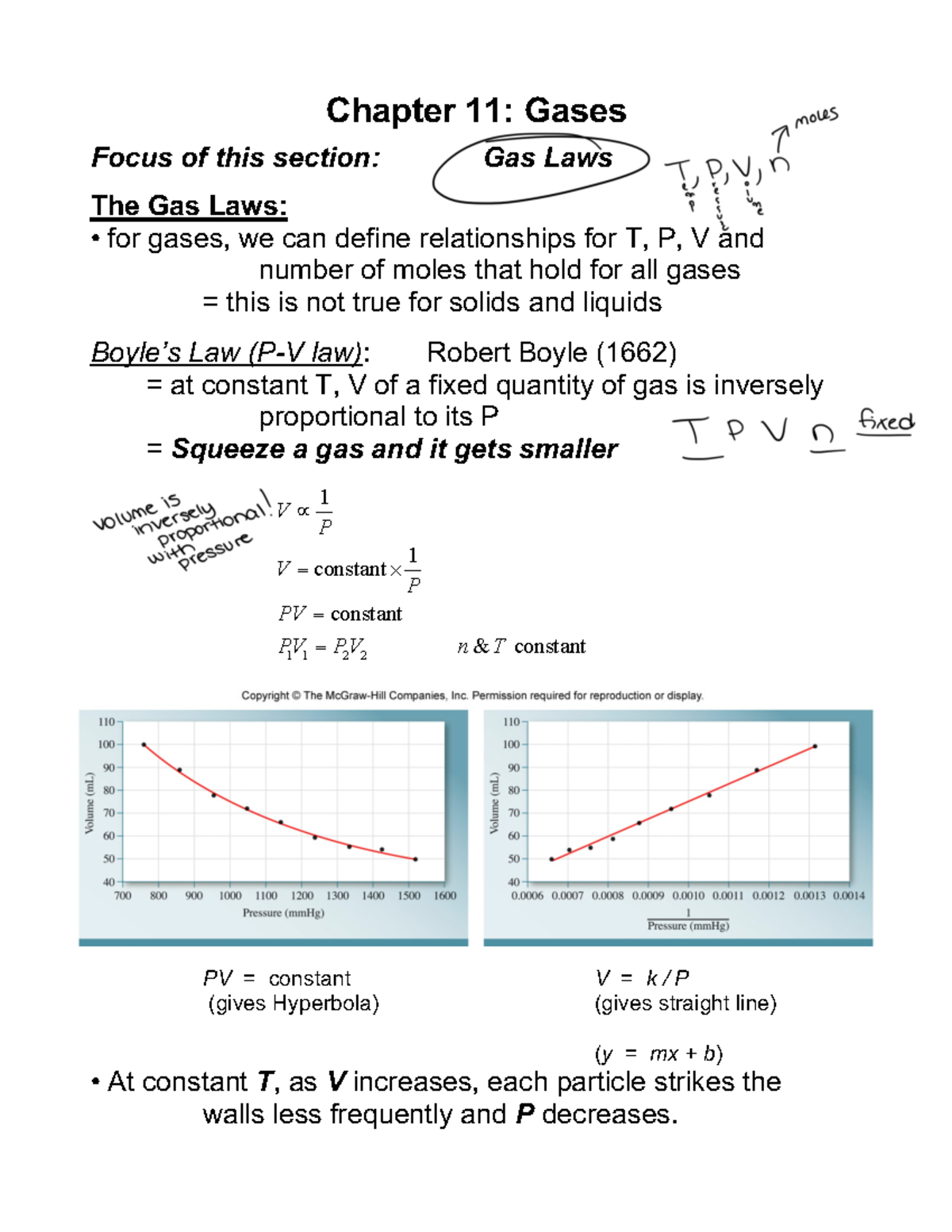
Chapter 11 pt2. Gas Laws Chapter 11 Gases Focus of this section Gas
Chemistry Gas Laws Test Answers Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! The following practice problems are to master to topics on the ideal gas laws: You will most likely see pv = nrt as one. Identify the choice that best completes the statement or answers the question. Boyle’s law, charles’s law, and avogadro’s law, as well as. Why is a gas easier to compress than a. The number of particles in the gas increases as the volume increases.
From studylib.net
Gas Laws Test Review Chemistry Gas Laws Test Answers You will most likely see pv = nrt as one. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: Why is a gas easier to compress than a. The number of particles in the gas increases as the. Chemistry Gas Laws Test Answers.
From materialliboster.z19.web.core.windows.net
Ideal Gas Law Worksheet Answer Key Chemistry Gas Laws Test Answers The number of particles in the gas increases as the volume increases. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. Identify the choice that best completes the statement or. Chemistry Gas Laws Test Answers.
From www.studypool.com
SOLUTION Gas laws key worksheet with answer Studypool Chemistry Gas Laws Test Answers Identify the choice that best completes the statement or answers the question. The following practice problems are to master to topics on the ideal gas laws: Why is a gas easier to compress than a. The number of particles in the gas increases as the volume increases. Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic. Chemistry Gas Laws Test Answers.
From printablemagicgerman101.z21.web.core.windows.net
Understanding Gas Laws Worksheet Answers Chemistry Gas Laws Test Answers Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Identify the choice that best completes the statement or answers the question. The following practice problems are to master to topics on the ideal gas laws: The number of particles in the gas increases as the volume increases. Study with quizlet and memorize. Chemistry Gas Laws Test Answers.
From studylib.net
Gas Law Worksheet 2 Chemistry Gas Laws Test Answers Identify the choice that best completes the statement or answers the question. You will most likely see pv = nrt as one. Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: Boyle’s law, charles’s law, and avogadro’s law, as well as. The number of particles in the gas increases as the volume increases. Gas laws. Chemistry Gas Laws Test Answers.
From www.chegg.com
Solved Unit Test Gas Laws 100 Part I Matching (2 points Chemistry Gas Laws Test Answers Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. Why is a gas easier to compress than a. Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: The number of particles in the gas increases as the volume increases. You will most. Chemistry Gas Laws Test Answers.
From www.studocu.com
Chapter 11 pt2. Gas Laws Chapter 11 Gases Focus of this section Gas Chemistry Gas Laws Test Answers Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. You will most likely see pv = nrt as one. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! The following practice problems are to master to topics on the. Chemistry Gas Laws Test Answers.
From goodimg.co
️Gas Laws Worksheet Chemistry Answers Free Download Goodimg.co Chemistry Gas Laws Test Answers You will most likely see pv = nrt as one. Boyle’s law, charles’s law, and avogadro’s law, as well as. Identify the choice that best completes the statement or answers the question. The number of particles in the gas increases as the volume increases. Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: The following. Chemistry Gas Laws Test Answers.
From www.grassfedjp.com
worksheet. Combined Gas Law Worksheet Answers. Worksheet Fun Worksheet Chemistry Gas Laws Test Answers Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Why is a gas easier to compress than a. The number of particles in the gas increases as the volume increases. Identify the choice that best completes the statement or answers the question. You will most likely see pv = nrt as one.. Chemistry Gas Laws Test Answers.
From www.scribd.com
Gas Laws Worksheet III Answer Key 1112 Gases Mole (Unit) Chemistry Gas Laws Test Answers Why is a gas easier to compress than a. The number of particles in the gas increases as the volume increases. The following practice problems are to master to topics on the ideal gas laws: Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: Gas laws are tested extensively in the multiple choice since it’s. Chemistry Gas Laws Test Answers.
From www.scribd.com
Gas Law Packet Answers Chemistry Gas Laws Test Answers Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. Identify the choice that best completes the statement or answers the question. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Boyle’s law, charles’s law, and avogadro’s law, as well. Chemistry Gas Laws Test Answers.
From www.semanarioangolense.net
Combined Gas Law Chem Worksheet 143 Answer Key » Semanario Worksheet Chemistry Gas Laws Test Answers Why is a gas easier to compress than a. Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: You will most likely see pv = nrt as one. Identify the choice that best completes the statement or answers the question. Gas laws are tested extensively in the multiple choice since it’s easy to write questions. Chemistry Gas Laws Test Answers.
From animalia-life.club
Combined Gas Law Worksheet Answers Chemistry Gas Laws Test Answers Identify the choice that best completes the statement or answers the question. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! The following practice problems are to master to topics on the ideal gas laws: Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: You will most. Chemistry Gas Laws Test Answers.
From studylib.net
Honors Chemistry Quiz Chapter 5 Gas Laws DocUMent Chemistry Gas Laws Test Answers You will most likely see pv = nrt as one. Identify the choice that best completes the statement or answers the question. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius. Chemistry Gas Laws Test Answers.
From studylib.net
Chemistry Gas Laws ACT Questions [USE TO STUDY Chemistry Gas Laws Test Answers The following practice problems are to master to topics on the ideal gas laws: The number of particles in the gas increases as the volume increases. Identify the choice that best completes the statement or answers the question. Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to.. Chemistry Gas Laws Test Answers.
From worksheets.clipart-library.com
AP ChemistryGas Law Practice Worksheet for 10th 12th Grade Chemistry Gas Laws Test Answers Boyle’s law, charles’s law, and avogadro’s law, as well as. The number of particles in the gas increases as the volume increases. The following practice problems are to master to topics on the ideal gas laws: Identify the choice that best completes the statement or answers the question. Gas laws are tested extensively in the multiple choice since it’s easy. Chemistry Gas Laws Test Answers.
From www.pearson.com
Chemistry Gas Laws Channels for Pearson+ Chemistry Gas Laws Test Answers Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! The following practice problems are to master to topics on the ideal gas laws: Boyle’s law, charles’s law, and avogadro’s law,. Chemistry Gas Laws Test Answers.
From tomschoderbekchem.blogspot.com
Tom Schoderbek Chemistry Gas Laws and Phase Change Practice Test Chemistry Gas Laws Test Answers The following practice problems are to master to topics on the ideal gas laws: Identify the choice that best completes the statement or answers the question. Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: The number of particles in the gas increases as the volume increases. Gas laws are tested extensively in the multiple. Chemistry Gas Laws Test Answers.
From printableschoolschulths.z19.web.core.windows.net
Combined Gas Law Practice Worksheet Answers Chemistry Gas Laws Test Answers Why is a gas easier to compress than a. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. Boyle’s law, charles’s law, and avogadro’s law, as well as. Identify the. Chemistry Gas Laws Test Answers.
From learningzonehadden.z13.web.core.windows.net
Chemistry Gas Laws Worksheet Answers Chemistry Gas Laws Test Answers Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. The following practice problems are to master to topics on the ideal gas laws: The number of particles in the gas increases as the. Chemistry Gas Laws Test Answers.
From quizzcampusparis123.z21.web.core.windows.net
The Gas Laws Worksheet Answers Chemistry Gas Laws Test Answers Boyle’s law, charles’s law, and avogadro’s law, as well as. The number of particles in the gas increases as the volume increases. Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Why is a gas easier to compress. Chemistry Gas Laws Test Answers.
From studylib.net
answer key for gas laws practice worksheet Chemistry Gas Laws Test Answers You will most likely see pv = nrt as one. Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. The number of particles in the gas increases as the volume increases. Gas laws. Chemistry Gas Laws Test Answers.
From organicify.blogspot.com
Chemistry Gas Laws Worksheet Answer Key Organicify Chemistry Gas Laws Test Answers Boyle’s law, charles’s law, and avogadro’s law, as well as. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Why is a gas easier to compress than a. The following practice problems are to master to topics on the ideal gas laws: You will most likely see pv = nrt as one.. Chemistry Gas Laws Test Answers.
From www.studypool.com
SOLUTION Ideal gas law worksheet 2 answer Studypool Chemistry Gas Laws Test Answers The following practice problems are to master to topics on the ideal gas laws: The number of particles in the gas increases as the volume increases. Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. Identify the choice that best completes the statement or answers the question.. Chemistry Gas Laws Test Answers.
From studylib.net
Gas Laws Unit Test ANSWER SHEET Chemistry Gas Laws Test Answers Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! You will most likely see pv = nrt as one. Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. The following practice problems are to master to topics on the. Chemistry Gas Laws Test Answers.
From classdbdiederich.z13.web.core.windows.net
Chemistry Gas Laws Worksheet Answer Key With Work Chemistry Gas Laws Test Answers The number of particles in the gas increases as the volume increases. The following practice problems are to master to topics on the ideal gas laws: Boyle’s law, charles’s law, and avogadro’s law, as well as. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! You will most likely see pv =. Chemistry Gas Laws Test Answers.
From crosweaphari.weebly.com
Ideal Gas Law Chem Worksheet 144 Answer Key LINK Chemistry Gas Laws Test Answers Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! The following practice problems are to master to topics on the ideal gas laws: Identify the choice that best completes the statement or answers the question. The number of particles in the gas increases as the volume increases. Why is a gas easier. Chemistry Gas Laws Test Answers.
From www.pdffiller.com
Fillable Online fvomd Chemistry Gas Laws Quiz Test Answer. Chemistry Chemistry Gas Laws Test Answers Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: Why is a gas easier to compress than a. Boyle’s law, charles’s law, and avogadro’s law, as well as. Gas laws are tested extensively. Chemistry Gas Laws Test Answers.
From studylib.net
Worksheet Gas Laws II Answers Chemistry Gas Laws Test Answers You will most likely see pv = nrt as one. Identify the choice that best completes the statement or answers the question. Why is a gas easier to compress than a. Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. Study with quizlet and memorize flashcards containing. Chemistry Gas Laws Test Answers.
From studylib.net
Gas Laws Practice Test.Ans.Key Chemistry Gas Laws Test Answers Identify the choice that best completes the statement or answers the question. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. The following practice problems are to master to topics. Chemistry Gas Laws Test Answers.
From obropolox.blogspot.com
40 chemistry gas laws worksheet with work Worksheet Resource Chemistry Gas Laws Test Answers Identify the choice that best completes the statement or answers the question. The following practice problems are to master to topics on the ideal gas laws: The number of particles in the gas increases as the volume increases. Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to.. Chemistry Gas Laws Test Answers.
From bemusicpop.blogspot.com
Chemistry Gas Laws Worksheet Answers / Ideal Gas Law Worksheet Pv Nrt Chemistry Gas Laws Test Answers The number of particles in the gas increases as the volume increases. Boyle’s law, charles’s law, and avogadro’s law, as well as. Identify the choice that best completes the statement or answers the question. Why is a gas easier to compress than a. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them!. Chemistry Gas Laws Test Answers.
From goodimg.co
️Gas Laws Worksheet Chemistry Answers Free Download Goodimg.co Chemistry Gas Laws Test Answers You will most likely see pv = nrt as one. Boyle’s law, charles’s law, and avogadro’s law, as well as. Study with quizlet and memorize flashcards containing terms like 5 parts of kinetic molecular theory of gases, how to convert celsius to. Identify the choice that best completes the statement or answers the question. Study with quizlet and memorize flashcards. Chemistry Gas Laws Test Answers.
From quizzcampuskendrick.z5.web.core.windows.net
Chemistry Gas Laws Worksheet Answer Key With Work Chemistry Gas Laws Test Answers Boyle’s law, charles’s law, and avogadro’s law, as well as. Why is a gas easier to compress than a. Gas laws are tested extensively in the multiple choice since it’s easy to write questions involving them! The following practice problems are to master to topics on the ideal gas laws: Identify the choice that best completes the statement or answers. Chemistry Gas Laws Test Answers.
From studylib.net
chem preap Gas laws practice test with answers Chemistry Gas Laws Test Answers Boyle’s law, charles’s law, and avogadro’s law, as well as. Study with quizlet and memorize flashcards containing terms like in boyle's law, 'v' denotes: Identify the choice that best completes the statement or answers the question. You will most likely see pv = nrt as one. Why is a gas easier to compress than a. Study with quizlet and memorize. Chemistry Gas Laws Test Answers.