Calorimetry Ice Melting In Water . the calorimetry calculator can help you solve complex calorimetry problems. liquid water has one of the highest specific heats known. This process is better known as melting, or heat of fusion,. the most common example is solid ice turning into liquid water. what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. Heat flow measurements can be made with either a constant. melting of ice occurs in two steps: First, the phase change occurs and solid (ice) transforms into liquid water at the melting. it's also known as enthalpy of fusion. Its units are usually joules per gram (j/g) or calories per gram (cal/g). It can analyze the heat exchange between up to 3 objects. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from www.chegg.com
Its units are usually joules per gram (j/g) or calories per gram (cal/g). This process is better known as melting, or heat of fusion,. It can analyze the heat exchange between up to 3 objects. the most common example is solid ice turning into liquid water. it's also known as enthalpy of fusion. Heat flow measurements can be made with either a constant. melting of ice occurs in two steps: what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. liquid water has one of the highest specific heats known. the calorimetry calculator can help you solve complex calorimetry problems.
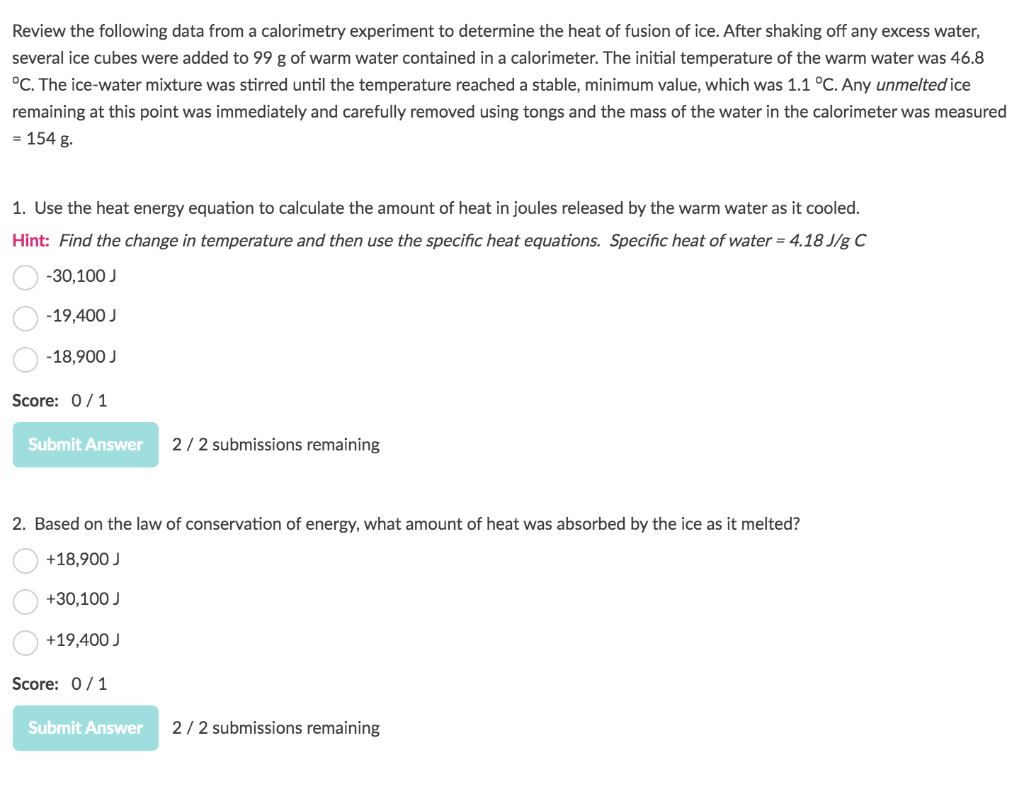
Solved Review the following data from a calorimetry
Calorimetry Ice Melting In Water what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. it's also known as enthalpy of fusion. the most common example is solid ice turning into liquid water. It can analyze the heat exchange between up to 3 objects. what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. liquid water has one of the highest specific heats known. Heat flow measurements can be made with either a constant. melting of ice occurs in two steps: Its units are usually joules per gram (j/g) or calories per gram (cal/g). First, the phase change occurs and solid (ice) transforms into liquid water at the melting. This process is better known as melting, or heat of fusion,. the calorimetry calculator can help you solve complex calorimetry problems. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From www.numerade.com
SOLVED Find the heat lost by the water, calorimeter cup (c = 0.21 Calorimetry Ice Melting In Water First, the phase change occurs and solid (ice) transforms into liquid water at the melting. what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. Its units are usually joules per gram (j/g) or calories per gram (cal/g). Heat flow measurements can be made with. Calorimetry Ice Melting In Water.
From www.tessshebaylo.com
Equation For Constant Volume Calorimetry Tessshebaylo Calorimetry Ice Melting In Water This process is better known as melting, or heat of fusion,. Its units are usually joules per gram (j/g) or calories per gram (cal/g). the calorimetry calculator can help you solve complex calorimetry problems. melting of ice occurs in two steps: liquid water has one of the highest specific heats known. First, the phase change occurs and. Calorimetry Ice Melting In Water.
From www.numerade.com
SOLVED Question 2 2 pts 5.0 g of ice is placed in a calorimeter Calorimetry Ice Melting In Water Heat flow measurements can be made with either a constant. it's also known as enthalpy of fusion. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. the calorimetry calculator can help you. Calorimetry Ice Melting In Water.
From spmchemistry.blog.onlinetuition.com.my
Three States of Matter Structured Question 4 SPM Chemistry Calorimetry Ice Melting In Water the most common example is solid ice turning into liquid water. Its units are usually joules per gram (j/g) or calories per gram (cal/g). it's also known as enthalpy of fusion. This process is better known as melting, or heat of fusion,. the calorimetry calculator can help you solve complex calorimetry problems. what is the final. Calorimetry Ice Melting In Water.
From www.numerade.com
SOLVED A Styrofoamcup calorimeter contains 300 g of water and a 45 g Calorimetry Ice Melting In Water Its units are usually joules per gram (j/g) or calories per gram (cal/g). melting of ice occurs in two steps: it's also known as enthalpy of fusion. the most common example is solid ice turning into liquid water. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Calorimetry Ice Melting In Water.
From www.thermopedia.com
SOLAR HEATING OF ICECOVERED LAKES AND ICE MELTING Calorimetry Ice Melting In Water liquid water has one of the highest specific heats known. Its units are usually joules per gram (j/g) or calories per gram (cal/g). the most common example is solid ice turning into liquid water. Heat flow measurements can be made with either a constant. it's also known as enthalpy of fusion. It can analyze the heat exchange. Calorimetry Ice Melting In Water.
From www.youtube.com
Final Temperature of Ice and Water Mixture How Many Grams of Ice Will Calorimetry Ice Melting In Water what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. it's also known as enthalpy of fusion. Its units are usually joules per gram (j/g) or calories per gram. Calorimetry Ice Melting In Water.
From www.numerade.com
SOLVED An Introduction to Everyday Calorimetry In this section; you Calorimetry Ice Melting In Water the most common example is solid ice turning into liquid water. what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. the calorimetry calculator can help you solve complex calorimetry problems. Its units are usually joules per gram (j/g) or calories per gram. Calorimetry Ice Melting In Water.
From www.youtube.com
Solving Complex Calorimetry Problems (Ice to Steam) YouTube Calorimetry Ice Melting In Water the most common example is solid ice turning into liquid water. it's also known as enthalpy of fusion. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the calorimetry calculator can help you solve complex calorimetry problems. what is the final temperature of the combined. Calorimetry Ice Melting In Water.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimetry Ice Melting In Water what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat flow measurements can be made with either a constant. melting of ice occurs. Calorimetry Ice Melting In Water.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Calorimetry Ice Melting In Water melting of ice occurs in two steps: Its units are usually joules per gram (j/g) or calories per gram (cal/g). liquid water has one of the highest specific heats known. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat flow measurements can be made with either. Calorimetry Ice Melting In Water.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry Ice Melting In Water First, the phase change occurs and solid (ice) transforms into liquid water at the melting. melting of ice occurs in two steps: Heat flow measurements can be made with either a constant. the most common example is solid ice turning into liquid water. it's also known as enthalpy of fusion. This process is better known as melting,. Calorimetry Ice Melting In Water.
From www.youtube.com
Calorimetry Aluminum Cup, Water, Ice, Lead YouTube Calorimetry Ice Melting In Water the most common example is solid ice turning into liquid water. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Its units are usually joules per gram (j/g) or calories per gram (cal/g). the calorimetry calculator can help you solve complex calorimetry problems. This process is better. Calorimetry Ice Melting In Water.
From www.chegg.com
Solved 5.2 CALORIMETRY 2) Example Melting Ice A flask Calorimetry Ice Melting In Water the most common example is solid ice turning into liquid water. it's also known as enthalpy of fusion. the calorimetry calculator can help you solve complex calorimetry problems. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. This process is better known as melting, or heat of fusion,. Its units are. Calorimetry Ice Melting In Water.
From www.youtube.com
Calorimetry STD 10 ICSE Calorimeter (ICE + WATER + VESSEL) numericals Calorimetry Ice Melting In Water liquid water has one of the highest specific heats known. melting of ice occurs in two steps: It can analyze the heat exchange between up to 3 objects. Heat flow measurements can be made with either a constant. it's also known as enthalpy of fusion. what is the final temperature of the combined system when 10.0. Calorimetry Ice Melting In Water.
From www.numerade.com
SOLVED A calorimeter contains 95 g of water at 25.0°C. A 5.0 g sample Calorimetry Ice Melting In Water the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. liquid water has one of the highest specific heats known. the most common example is solid ice turning into liquid water. it's also known as enthalpy of fusion. melting of ice occurs in two. Calorimetry Ice Melting In Water.
From saylordotorg.github.io
Calorimetry Calorimetry Ice Melting In Water the most common example is solid ice turning into liquid water. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This process is better known as melting, or heat of fusion,. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. It can. Calorimetry Ice Melting In Water.
From slideplayer.com
Make a modelmelting ice on two blocks ppt download Calorimetry Ice Melting In Water Its units are usually joules per gram (j/g) or calories per gram (cal/g). a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the most common example is solid ice turning into liquid water. the calorimetry calculator can help you solve complex calorimetry problems. melting of ice. Calorimetry Ice Melting In Water.
From www.toppr.com
50g ice at 0^0C is dropped into a calorimeter containing 100g water at Calorimetry Ice Melting In Water what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. liquid water has one of the highest specific heats known. Its units are usually joules per gram (j/g) or calories per gram (cal/g). it's also known as enthalpy of fusion. Heat flow measurements. Calorimetry Ice Melting In Water.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Calorimetry Ice Melting In Water melting of ice occurs in two steps: a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. it's also known as enthalpy of fusion. the calorimetry calculator can help you solve complex calorimetry problems. First, the phase change occurs and solid (ice) transforms into liquid water at. Calorimetry Ice Melting In Water.
From www.researchgate.net
Adiabatic calorimeter (a) schematic layout of calorimeter, ice Calorimetry Ice Melting In Water what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. It can analyze the heat exchange between up to 3 objects. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. a calorimeter is a device used to measure. Calorimetry Ice Melting In Water.
From www.youtube.com
CALORIMETRY PART 9 ICE WATER MIXTURE YouTube Calorimetry Ice Melting In Water what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. liquid water has one of the highest specific heats known. it's also known as enthalpy of fusion. This process is better known as melting, or heat of fusion,. melting of ice occurs. Calorimetry Ice Melting In Water.
From www.numerade.com
SOLVED AP Chemistry Review Questions Integrating Content; Inquiry and Calorimetry Ice Melting In Water liquid water has one of the highest specific heats known. the calorimetry calculator can help you solve complex calorimetry problems. what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. Heat flow measurements can be made with either a constant. a calorimeter. Calorimetry Ice Melting In Water.
From www.researchgate.net
Schematic representation of thermal events detectable by differential Calorimetry Ice Melting In Water the calorimetry calculator can help you solve complex calorimetry problems. liquid water has one of the highest specific heats known. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. the most common example is solid ice turning into liquid water. a calorimeter is a device used to measure the amount. Calorimetry Ice Melting In Water.
From www.numerade.com
SOLVED The following data was recorded mass of calorimeter, mass of Calorimetry Ice Melting In Water Heat flow measurements can be made with either a constant. it's also known as enthalpy of fusion. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the most common example is solid ice turning into liquid water. First, the phase change occurs and solid (ice) transforms into. Calorimetry Ice Melting In Water.
From www.researchgate.net
Adiabatic calorimeter (a) schematic layout of calorimeter, ice Calorimetry Ice Melting In Water It can analyze the heat exchange between up to 3 objects. melting of ice occurs in two steps: Its units are usually joules per gram (j/g) or calories per gram (cal/g). a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This process is better known as melting, or. Calorimetry Ice Melting In Water.
From www.updateans.com
Update ANS 80 g of water a 30°C are poured on a large block of ice at Calorimetry Ice Melting In Water First, the phase change occurs and solid (ice) transforms into liquid water at the melting. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the most common example is solid ice turning into liquid water. This process is better known as melting, or heat of fusion,. what. Calorimetry Ice Melting In Water.
From www.chegg.com
Solved Review the following data from a calorimetry Calorimetry Ice Melting In Water It can analyze the heat exchange between up to 3 objects. Its units are usually joules per gram (j/g) or calories per gram (cal/g). liquid water has one of the highest specific heats known. This process is better known as melting, or heat of fusion,. the calorimetry calculator can help you solve complex calorimetry problems. a calorimeter. Calorimetry Ice Melting In Water.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Ice Melting In Water what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. First, the phase change occurs and solid (ice) transforms into liquid water at the melting.. Calorimetry Ice Melting In Water.
From www.youtube.com
How do you measure DeltaH? calorimetry YouTube Calorimetry Ice Melting In Water it's also known as enthalpy of fusion. Its units are usually joules per gram (j/g) or calories per gram (cal/g). a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. liquid water has one of the highest specific heats known. what is the final temperature of the. Calorimetry Ice Melting In Water.
From www.numerade.com
Using the values for the heat of fusion, specific heat of water, and/or Calorimetry Ice Melting In Water what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. liquid water has one of the highest specific heats known. First, the phase change. Calorimetry Ice Melting In Water.
From www.jpl.nasa.gov
Educator Guide Melting Ice Experiment NASA/JPL Edu Calorimetry Ice Melting In Water This process is better known as melting, or heat of fusion,. what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. the calorimetry calculator can help you solve complex calorimetry problems. liquid water has one of the highest specific heats known. It can. Calorimetry Ice Melting In Water.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimetry Ice Melting In Water what is the final temperature of the combined system when 10.0 g of ice is placed into 50.0 g of water at 32.0∘ c?. This process is better known as melting, or heat of fusion,. Its units are usually joules per gram (j/g) or calories per gram (cal/g). Heat flow measurements can be made with either a constant. . Calorimetry Ice Melting In Water.
From www.slideshare.net
Priestley & lavoisier 112 Calorimetry Ice Melting In Water Heat flow measurements can be made with either a constant. the calorimetry calculator can help you solve complex calorimetry problems. the most common example is solid ice turning into liquid water. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. a calorimeter is a device used to measure the amount of. Calorimetry Ice Melting In Water.
From lefafrankmackenzie.blogspot.com
Specific Heat Capacity of Ice Frank Mackenzie Calorimetry Ice Melting In Water the most common example is solid ice turning into liquid water. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Its units are usually joules per gram (j/g) or calories per gram (cal/g). This process is better known as melting, or heat of fusion,. melting of ice. Calorimetry Ice Melting In Water.