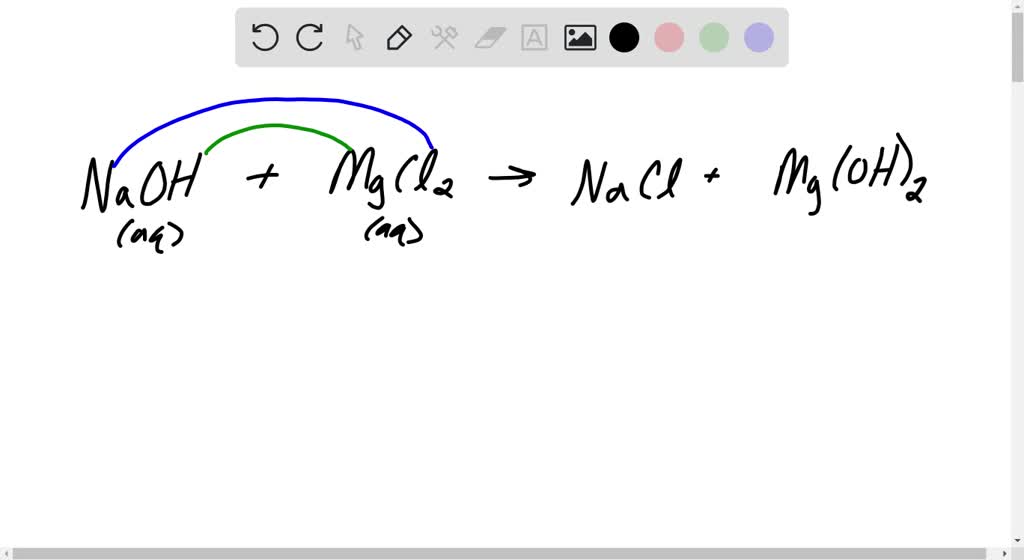
Magnesium Hydroxide + Chloric Acid Formula . in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. Magnesium hydroxide is a strong base while chloric acid is a strong acid. Chloric acid + magnesium hydroxide = magnesium chlorate + water. there are three main steps for writing the net ionic equation for mg + hcl. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. Enter an equation of an ionic chemical equation and press the balance button. calculate net ionic equation. the balanced chemical equation is: mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation.
from www.numerade.com
mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Magnesium hydroxide is a strong base while chloric acid is a strong acid. calculate net ionic equation. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. Chloric acid + magnesium hydroxide = magnesium chlorate + water. there are three main steps for writing the net ionic equation for mg + hcl. Enter an equation of an ionic chemical equation and press the balance button. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. the balanced chemical equation is:
SOLVED What will cause more Magnesium to be dissolved in a solution of
Magnesium Hydroxide + Chloric Acid Formula there are three main steps for writing the net ionic equation for mg + hcl. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Magnesium hydroxide is a strong base while chloric acid is a strong acid. there are three main steps for writing the net ionic equation for mg + hcl. calculate net ionic equation. Chloric acid + magnesium hydroxide = magnesium chlorate + water. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. the balanced chemical equation is: in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. Enter an equation of an ionic chemical equation and press the balance button.
From www.numerade.com
SOLVED What will cause more Magnesium to be dissolved in a solution of Magnesium Hydroxide + Chloric Acid Formula Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. Enter an equation of an ionic chemical equation and press the balance button. calculate net ionic equation. Magnesium hydroxide is a strong base while chloric acid is a strong acid. the balanced chemical equation is: Chloric acid + magnesium hydroxide = magnesium chlorate + water. mg(oh)2(s) +2hcl(aq) →. Magnesium Hydroxide + Chloric Acid Formula.
From www.garrisonminerals.com
How Magnesium Hydroxide is Made Magnesium Hydroxide + Chloric Acid Formula Chloric acid + magnesium hydroxide = magnesium chlorate + water. the balanced chemical equation is: Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. calculate net ionic equation. there are three main steps for writing the net ionic equation for mg + hcl. Magnesium hydroxide is a strong base while chloric acid is a strong acid. in this video we'll. Magnesium Hydroxide + Chloric Acid Formula.
From www.tes.com
Ionic Equations Acids and Salts Edexcel 91 Combined Science Teaching Magnesium Hydroxide + Chloric Acid Formula Chloric acid + magnesium hydroxide = magnesium chlorate + water. Enter an equation of an ionic chemical equation and press the balance button. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. there are three main steps for writing the. Magnesium Hydroxide + Chloric Acid Formula.
From www.dreamstime.com
Dihydroxide Stock Illustrations 2 Dihydroxide Stock Illustrations Magnesium Hydroxide + Chloric Acid Formula Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. Enter an equation of an ionic chemical equation and press the balance button. Chloric acid + magnesium hydroxide = magnesium chlorate + water. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. Magnesium hydroxide is a strong base while chloric acid is a strong acid. there are three main steps for writing the net. Magnesium Hydroxide + Chloric Acid Formula.
From mungfali.com
PPT Section 9.1 Naming Ions PowerPoint Presentation Magnesium Hydroxide + Chloric Acid Formula Magnesium hydroxide is a strong base while chloric acid is a strong acid. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. calculate net ionic equation. there are three main steps for writing the net ionic equation for mg + hcl. Mg_((s))+. Magnesium Hydroxide + Chloric Acid Formula.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Hydroxide + Chloric Acid Formula Enter an equation of an ionic chemical equation and press the balance button. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Magnesium hydroxide is a strong base while chloric acid is a strong acid. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. calculate net ionic equation. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. the balanced. Magnesium Hydroxide + Chloric Acid Formula.
From mavink.com
Magnesium And Hcl Reaction Magnesium Hydroxide + Chloric Acid Formula Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. Magnesium hydroxide is a strong base while chloric acid is a strong acid. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Enter an equation of an ionic chemical equation and press the balance button. in this video we'll balance the equation. Magnesium Hydroxide + Chloric Acid Formula.
From www.chemicalslearning.com
Magnesium Chloride Formula, Properties and Uses Magnesium Hydroxide + Chloric Acid Formula Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Magnesium hydroxide is a strong base while chloric acid is a strong acid. Chloric acid + magnesium hydroxide = magnesium chlorate + water. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. calculate net ionic. Magnesium Hydroxide + Chloric Acid Formula.
From www.youtube.com
Magnesium Hydroxide and Hydrochloric Acid Reaction with Universal Magnesium Hydroxide + Chloric Acid Formula calculate net ionic equation. there are three main steps for writing the net ionic equation for mg + hcl. the balanced chemical equation is: Chloric acid + magnesium hydroxide = magnesium chlorate + water. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. Magnesium hydroxide. Magnesium Hydroxide + Chloric Acid Formula.
From www.bartleby.com
Answered Magnesium metal reacts with… bartleby Magnesium Hydroxide + Chloric Acid Formula there are three main steps for writing the net ionic equation for mg + hcl. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. the balanced chemical equation is: Chloric acid + magnesium hydroxide = magnesium chlorate + water. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. calculate net ionic equation. Enter an. Magnesium Hydroxide + Chloric Acid Formula.
From brainly.com
3.) magnesium reacts with Hydro chloric acid according to the following Magnesium Hydroxide + Chloric Acid Formula mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. calculate net ionic equation. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. Enter an equation of an ionic chemical equation and press the balance button. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. Magnesium hydroxide is a strong base while chloric acid. Magnesium Hydroxide + Chloric Acid Formula.
From solvedlib.com
Magnesium hydroxide reacts with hydrochloric acid to … SolvedLib Magnesium Hydroxide + Chloric Acid Formula Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Magnesium hydroxide is a strong base while chloric acid is a strong acid. Enter an equation of an ionic chemical equation and press the balance button. there are three main steps for writing the net ionic equation for mg + hcl. . Magnesium Hydroxide + Chloric Acid Formula.
From www.youtube.com
How to Write the Formula for Magnesium hydroxide YouTube Magnesium Hydroxide + Chloric Acid Formula the balanced chemical equation is: Enter an equation of an ionic chemical equation and press the balance button. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. Magnesium hydroxide is a strong base while chloric acid is a strong acid. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Chloric acid. Magnesium Hydroxide + Chloric Acid Formula.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Magnesium Hydroxide + Chloric Acid Formula Enter an equation of an ionic chemical equation and press the balance button. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Magnesium hydroxide is a strong base while chloric acid is a strong acid. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. the balanced chemical equation is:. Magnesium Hydroxide + Chloric Acid Formula.
From webapi.bu.edu
😝 Magnesium plus hydrochloric acid. What products does magnesium and Magnesium Hydroxide + Chloric Acid Formula Chloric acid + magnesium hydroxide = magnesium chlorate + water. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. calculate net ionic equation. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. Enter an equation of an ionic chemical equation and press the balance button. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical. Magnesium Hydroxide + Chloric Acid Formula.
From mavink.com
Magnesium Reacts With Hydrochloric Acid Magnesium Hydroxide + Chloric Acid Formula Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. the balanced chemical equation is: Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. Enter an equation of an ionic chemical equation and press the balance button. there are three main steps for writing the net ionic equation for mg + hcl. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical. Magnesium Hydroxide + Chloric Acid Formula.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Hydroxide + Chloric Acid Formula Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. Chloric acid + magnesium hydroxide = magnesium chlorate + water. the balanced chemical equation is: Magnesium hydroxide is a strong base while chloric acid is a strong acid. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. Enter. Magnesium Hydroxide + Chloric Acid Formula.
From www.chegg.com
Solved 20 Write the balanced equation for the reaction of Magnesium Hydroxide + Chloric Acid Formula in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. Enter an equation of an ionic chemical equation and press the balance button. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. the balanced chemical equation is: calculate net ionic equation. there are three main steps for writing the net ionic. Magnesium Hydroxide + Chloric Acid Formula.
From www.bartleby.com
Answered 10. Write the correct name or formula… bartleby Magnesium Hydroxide + Chloric Acid Formula Chloric acid + magnesium hydroxide = magnesium chlorate + water. calculate net ionic equation. the balanced chemical equation is: there are three main steps for writing the net ionic equation for mg + hcl. Enter an equation of an ionic chemical equation and press the balance button. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced. Magnesium Hydroxide + Chloric Acid Formula.
From sujatanutripharma.com
Magnesium Hydroxide Sujata Nutri Pharma Magnesium Hydroxide + Chloric Acid Formula mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Chloric acid + magnesium hydroxide = magnesium chlorate + water. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. Magnesium hydroxide is a strong base while chloric acid is a strong acid. calculate net ionic equation. the balanced chemical equation is: Enter an equation of an. Magnesium Hydroxide + Chloric Acid Formula.
From www.chegg.com
3. The reaction between magnesium hydroxide and Magnesium Hydroxide + Chloric Acid Formula calculate net ionic equation. Chloric acid + magnesium hydroxide = magnesium chlorate + water. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. there are three main steps for writing the net ionic equation for mg + hcl. Magnesium hydroxide is a strong base while chloric. Magnesium Hydroxide + Chloric Acid Formula.
From molekula.com
Purchase Magnesium hydroxide [1309428] online • Catalog • Molekula Group Magnesium Hydroxide + Chloric Acid Formula Chloric acid + magnesium hydroxide = magnesium chlorate + water. there are three main steps for writing the net ionic equation for mg + hcl. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. Enter an equation of an ionic chemical equation and press the balance button. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Magnesium hydroxide is a. Magnesium Hydroxide + Chloric Acid Formula.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Magnesium Hydroxide + Chloric Acid Formula calculate net ionic equation. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. the balanced chemical equation is: Magnesium hydroxide is a strong base while chloric acid is a strong acid. Chloric acid + magnesium hydroxide = magnesium chlorate + water. Enter an equation of an ionic chemical equation and press the balance button. in this video we'll balance the equation. Magnesium Hydroxide + Chloric Acid Formula.
From www.youtube.com
Reaction between Magnesium and Water (Mg + H2O) YouTube Magnesium Hydroxide + Chloric Acid Formula in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. there are three main steps for writing the net ionic equation for mg + hcl. Enter an equation of an ionic chemical equation and press the balance button. the balanced. Magnesium Hydroxide + Chloric Acid Formula.
From www.fishersci.de
Magnesiumhydroxid, 95 , rein, Thermo Scientific Chemicals Fisher Magnesium Hydroxide + Chloric Acid Formula Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. Chloric acid + magnesium hydroxide = magnesium chlorate + water. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. the balanced chemical equation is: there are three main steps for writing the net ionic equation for mg + hcl. Enter an equation of an ionic chemical. Magnesium Hydroxide + Chloric Acid Formula.
From www.numerade.com
SOLVED Solid magnesium hydroxide into gaseous water and Magnesium Hydroxide + Chloric Acid Formula the balanced chemical equation is: Magnesium hydroxide is a strong base while chloric acid is a strong acid. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. calculate net ionic equation. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. there are three main steps for writing the net ionic equation for mg + hcl. Enter an equation. Magnesium Hydroxide + Chloric Acid Formula.
From www.numerade.com
SOLVED Write the molecular, complete ionic, and net ionic equation for Magnesium Hydroxide + Chloric Acid Formula Chloric acid + magnesium hydroxide = magnesium chlorate + water. Magnesium hydroxide is a strong base while chloric acid is a strong acid. there are three main steps for writing the net ionic equation for mg + hcl. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. Enter an equation of. Magnesium Hydroxide + Chloric Acid Formula.
From www.tessshebaylo.com
Chemical Equation For Sodium Hydrogen Carbonate And Hydrochloric Acid Magnesium Hydroxide + Chloric Acid Formula calculate net ionic equation. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. Enter an equation of an ionic chemical equation and press the balance button. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the.. Magnesium Hydroxide + Chloric Acid Formula.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium Hydroxide + Chloric Acid Formula there are three main steps for writing the net ionic equation for mg + hcl. Enter an equation of an ionic chemical equation and press the balance button. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Magnesium hydroxide is a strong base while chloric acid is a strong acid. the balanced chemical equation is:. Magnesium Hydroxide + Chloric Acid Formula.
From www.numerade.com
SOLVED Write the balanced equation for Magnesium nitride + water Magnesium Hydroxide + Chloric Acid Formula in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Enter an equation of an ionic chemical equation and press the balance button. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. calculate net ionic equation. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a.. Magnesium Hydroxide + Chloric Acid Formula.
From www.tessshebaylo.com
Sodium Bicarbonate And Hydrochloric Acid Net Ionic Equation Tessshebaylo Magnesium Hydroxide + Chloric Acid Formula Chloric acid + magnesium hydroxide = magnesium chlorate + water. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. calculate net ionic equation. the balanced chemical equation is: Enter an equation of an ionic chemical equation and press the balance button. in this video we'll. Magnesium Hydroxide + Chloric Acid Formula.
From www.chegg.com
Magnesium metal reacts with hydrochloric acid Magnesium Hydroxide + Chloric Acid Formula Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. calculate net ionic equation. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. Enter an equation of an ionic chemical equation and press the balance button. there are three main steps for writing the net ionic equation for mg + hcl. in this video we'll balance the equation mg (oh)2 + hcl. Magnesium Hydroxide + Chloric Acid Formula.
From mammothmemory.net
Similarly magnesium and hydrochloric acid is a slow reaction Magnesium Hydroxide + Chloric Acid Formula there are three main steps for writing the net ionic equation for mg + hcl. the balanced chemical equation is: calculate net ionic equation. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. Magnesium hydroxide is a strong base while chloric acid is a strong acid. Hclo3 + mg(oh)2 =. Magnesium Hydroxide + Chloric Acid Formula.
From www.scibond.com
How to represent a chemical reaction? SciBond Magnesium Hydroxide + Chloric Acid Formula in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. Enter an equation of an ionic chemical equation and press the balance button. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Magnesium hydroxide is a strong. Magnesium Hydroxide + Chloric Acid Formula.
From www.chegg.com
Solved 1 6. (25pts)Magnesium Magnesium Hydroxide + Chloric Acid Formula there are three main steps for writing the net ionic equation for mg + hcl. calculate net ionic equation. Hclo3 + mg(oh)2 = mg(clo3)2 + h2o is a. in this video we'll balance the equation mg (oh)2 + hcl = mgcl2 + h2o. mg(oh)2(s) +2hcl(aq) → mgcl2(aq) + 2h2o(l) for a balanced chemical equation. Enter an. Magnesium Hydroxide + Chloric Acid Formula.