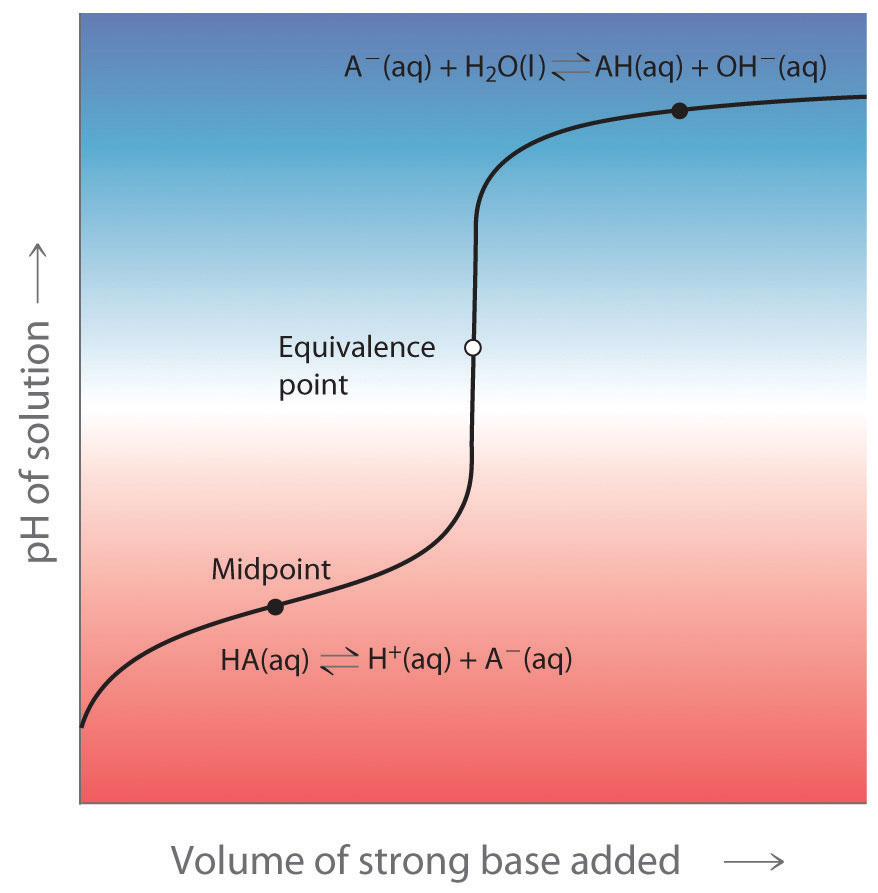
What Is A Neutral Buffer Zone . A buffering agent is a weak acid or weak base that helps maintain the ph of an. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. understand the relationship between the titration curve of a weak acid or base and buffers; Understand the use of indicators. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is an aqueous solution that has a highly stable ph. what is a buffer solution? It is able to neutralize small.
from exosxgjvz.blob.core.windows.net
It is able to neutralize small. Understand the use of indicators. a buffer is an aqueous solution that has a highly stable ph. understand the relationship between the titration curve of a weak acid or base and buffers; what is a buffer solution? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a. A buffering agent is a weak acid or weak base that helps maintain the ph of an. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it.
Weak Acid Titration Curve Buffer Region at Paula Rivera blog
What Is A Neutral Buffer Zone Understand the use of indicators. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffering agent is a weak acid or weak base that helps maintain the ph of an. understand the relationship between the titration curve of a weak acid or base and buffers; a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a. a buffer is an aqueous solution that has a highly stable ph. Understand the use of indicators. what is a buffer solution? It is able to neutralize small.
From www.springfieldmo.gov
Regulations Springfield, MO Official site What Is A Neutral Buffer Zone A buffering agent is a weak acid or weak base that helps maintain the ph of an. Understand the use of indicators. what is a buffer solution? a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. a. What Is A Neutral Buffer Zone.
From www.luxwisp.com
What is a Buffer Zone in Real Estate Luxwisp What Is A Neutral Buffer Zone understand the relationship between the titration curve of a weak acid or base and buffers; what is a buffer solution? a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a. a buffer is an aqueous solution that has. What Is A Neutral Buffer Zone.
From www.researchgate.net
Definition of buffer zones for the impact assessment of houses by What Is A Neutral Buffer Zone a buffer is an aqueous solution that has a highly stable ph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Understand the use of indicators. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid). What Is A Neutral Buffer Zone.
From norezone.org
What is a Buffer Zone? North Oak Cliff Residents for Responsible What Is A Neutral Buffer Zone a buffer is an aqueous solution that has a highly stable ph. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Understand the use of indicators. a buffer is a solution that can resist ph change upon the addition of an acidic or basic. What Is A Neutral Buffer Zone.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog What Is A Neutral Buffer Zone what is a buffer solution? a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a. understand the relationship between the titration curve of a weak acid or base and buffers; a mixture of a weak acid and its. What Is A Neutral Buffer Zone.
From www.dw.com
Buffer zone in Idlib A ray of hope in Syria Middle East News and What Is A Neutral Buffer Zone a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffering agent is a weak acid or weak base. What Is A Neutral Buffer Zone.
From www.researchgate.net
Buffer map of communities 5km from the airport. Download Scientific What Is A Neutral Buffer Zone A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffering agent is a weak acid or weak base that helps maintain the ph of an. what is a buffer solution? It is able to neutralize small. Understand the use of indicators. a buffer. What Is A Neutral Buffer Zone.
From www.researchgate.net
Biosphere reserve zonation in core area, buffer zone, and transition What Is A Neutral Buffer Zone a buffer is an aqueous solution that has a highly stable ph. Understand the use of indicators. It is able to neutralize small. understand the relationship between the titration curve of a weak acid or base and buffers; a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its. What Is A Neutral Buffer Zone.
From www.researchgate.net
(PDF) Accessibility of Health Care Institutions A Case Study by Using GIS What Is A Neutral Buffer Zone Understand the use of indicators. It is able to neutralize small. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components.. What Is A Neutral Buffer Zone.
From www.researchgate.net
Estimated riparian buffer zones (RBZs) for perennial, ephemeral, and What Is A Neutral Buffer Zone a buffer is an aqueous solution that has a highly stable ph. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. understand the relationship between the titration curve of a weak acid or base and buffers; . What Is A Neutral Buffer Zone.
From www.russellconveyor.com
Buffer Zone Basics the Simplified Truth Russell Conveyor What Is A Neutral Buffer Zone A buffering agent is a weak acid or weak base that helps maintain the ph of an. Understand the use of indicators. a buffer is an aqueous solution that has a highly stable ph. what is a buffer solution? understand the relationship between the titration curve of a weak acid or base and buffers; a buffer. What Is A Neutral Buffer Zone.
From exosxgjvz.blob.core.windows.net
Weak Acid Titration Curve Buffer Region at Paula Rivera blog What Is A Neutral Buffer Zone A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is an aqueous solution that has a highly stable ph. a mixture of. What Is A Neutral Buffer Zone.
From www.trafficsafetystore.com
fig6h_31 Traffic Safety Resource Center What Is A Neutral Buffer Zone what is a buffer solution? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. understand the relationship between the titration curve of a weak acid or base and buffers; a buffer is an aqueous solution that has a highly stable ph. Understand the use of indicators.. What Is A Neutral Buffer Zone.
From www.researchgate.net
Buffer zone analysis. Anomalous DREP values are in black. Blue line What Is A Neutral Buffer Zone understand the relationship between the titration curve of a weak acid or base and buffers; Understand the use of indicators. what is a buffer solution? a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a. a buffer is. What Is A Neutral Buffer Zone.
From gis.stackexchange.com
arcpy Create area buffers that are limited by a coastline What Is A Neutral Buffer Zone what is a buffer solution? a buffer is an aqueous solution that has a highly stable ph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Understand the use of indicators. understand the relationship between the titration curve of a weak acid or base and buffers;. What Is A Neutral Buffer Zone.
From www.researchgate.net
Illustration of buffer zones used in this study. The June's buffer zone What Is A Neutral Buffer Zone a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. understand the relationship between the titration curve of a weak acid or base and buffers; A buffering agent is a weak acid or weak base that helps maintain the ph of an. a mixture of a weak acid. What Is A Neutral Buffer Zone.
From english.mathrubhumi.com
Buffer zone Kerala to make available enlisted survey numbers within a What Is A Neutral Buffer Zone a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Understand the use of indicators. a mixture. What Is A Neutral Buffer Zone.
From www.researchgate.net
Buffer zone map of main buildings. Download Scientific Diagram What Is A Neutral Buffer Zone A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is able to neutralize small. Understand the use of indicators. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. understand the relationship between the. What Is A Neutral Buffer Zone.
From thinkwildlifefoundation.com
What are Buffer Zones and why are they important? What Is A Neutral Buffer Zone Understand the use of indicators. It is able to neutralize small. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer is an aqueous. What Is A Neutral Buffer Zone.
From newyorkparkingticket.com
Can you Distinguish a Safety Zone from a Buffer Zone? What Is A Neutral Buffer Zone a buffer is an aqueous solution that has a highly stable ph. what is a buffer solution? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. a mixture of a weak acid and its conjugate base (or a mixture of. What Is A Neutral Buffer Zone.
From www.frontiersin.org
Frontiers Application of riparian buffer zone in agricultural non What Is A Neutral Buffer Zone a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Understand the use of indicators. It is able to neutralize small.. What Is A Neutral Buffer Zone.
From www.semanticscholar.org
[PDF] The Uniqueness of Erbil Citadel Buffer Zone as Compared to the What Is A Neutral Buffer Zone a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffering agent is a weak acid or weak base that helps maintain the ph of an. a mixture of a weak acid and its conjugate base (or a. What Is A Neutral Buffer Zone.
From www.researchgate.net
(PDF) Preliminary guideline for the determination of buffer zones for What Is A Neutral Buffer Zone Understand the use of indicators. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffering agent is a weak acid or weak base that helps maintain the ph of an. what is a buffer solution? understand the relationship between the titration curve of a weak acid. What Is A Neutral Buffer Zone.
From www.fishersci.com
StatLab Neutral Buffered Formalin (NBF) 10, pH 7Histology and What Is A Neutral Buffer Zone A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. understand the relationship between the titration curve of a weak acid or base and buffers; a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate. What Is A Neutral Buffer Zone.
From www.researchgate.net
Buffer areas for 50 m and 200 m. Download Scientific Diagram What Is A Neutral Buffer Zone a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffering agent is a weak acid or weak base that helps maintain the ph of an. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it.. What Is A Neutral Buffer Zone.
From newyorkparkingticket.com
Can you Distinguish a Safety Zone from a Buffer Zone? What Is A Neutral Buffer Zone a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. a buffer is an aqueous solution that has a highly stable ph. a buffer is a solution that can resist ph change upon the addition of an acidic. What Is A Neutral Buffer Zone.
From www.researchgate.net
Buffer zones from the major roads Download Scientific Diagram What Is A Neutral Buffer Zone understand the relationship between the titration curve of a weak acid or base and buffers; a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Understand the use of indicators. It is able to neutralize small. a buffer. What Is A Neutral Buffer Zone.
From www.youtube.com
NCEA L3 Chem Buffers, Buffer Region & Preparation of Buffer Solutions What Is A Neutral Buffer Zone a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. It is able to neutralize small. what is a buffer solution? a buffer is an aqueous solution that has a highly stable ph. Understand the use of indicators.. What Is A Neutral Buffer Zone.
From voi.id
Apa Itu Buffer Zone dan Fungsinya untuk Kawasan TBBM What Is A Neutral Buffer Zone a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. It is able to neutralize small. A buffering agent is a weak acid or weak base that helps maintain the ph of an. understand the relationship between the titration. What Is A Neutral Buffer Zone.
From www.researchgate.net
Map of buffer zones (6.0 km) around the rivers Download Scientific What Is A Neutral Buffer Zone A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a. A buffering agent is a weak acid or. What Is A Neutral Buffer Zone.
From edu.svet.gob.gt
10 Neutral Buffered Formalin Ph edu.svet.gob.gt What Is A Neutral Buffer Zone a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer is an aqueous solution. What Is A Neutral Buffer Zone.
From www.vedantu.com
Core zone, buffer zone and manipulation zone are found in(a) Tiger What Is A Neutral Buffer Zone Understand the use of indicators. what is a buffer solution? It is able to neutralize small. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer solution is one which resists changes in ph when small quantities. What Is A Neutral Buffer Zone.
From www.researchgate.net
Rivers and 25 m buffer zone in IYTE Download Scientific Diagram What Is A Neutral Buffer Zone a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Understand the use of indicators. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is able to neutralize small. a mixture of a weak. What Is A Neutral Buffer Zone.
From www.catchments.ie
Better buffer Zones Catchments.ie Catchments.ie What Is A Neutral Buffer Zone what is a buffer solution? a buffer is an aqueous solution that has a highly stable ph. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. a buffer is a solution that can resist ph change. What Is A Neutral Buffer Zone.
From www.chegg.com
Solved Consider the following questions about buffer What Is A Neutral Buffer Zone Understand the use of indicators. a buffer is an aqueous solution that has a highly stable ph. understand the relationship between the titration curve of a weak acid or base and buffers; A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a mixture. What Is A Neutral Buffer Zone.