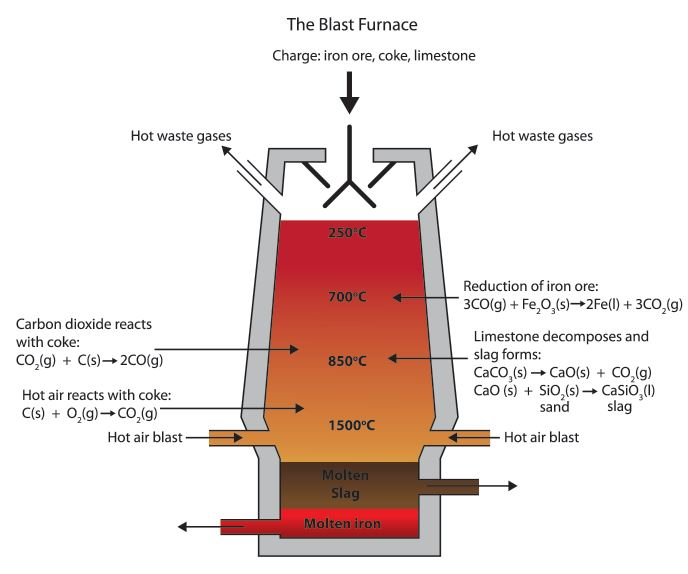
Iron Is Extracted From Its Ore Using A Blast Furnace Igcse . iron is extracted from iron ore in a huge container called a blast furnace. in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. Two of the impurities are. Such as haematite contain iron (iii) oxide, fe 2 o 3. 5 iron is extracted from its ore using a blast furnace. Fe2o3 + 3co → 2fe +. (a) in the blast furnace, coke burns in oxygen to produce heat energy and carbon dioxide. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. the process happens in a blast furnace. The oxygen must be removed from the iron. (c) the iron from the blast furnace contains about 10% by mass of impurities.
from mavink.com
in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. 5 iron is extracted from its ore using a blast furnace. (c) the iron from the blast furnace contains about 10% by mass of impurities. the process happens in a blast furnace. Two of the impurities are. Such as haematite contain iron (iii) oxide, fe 2 o 3. Fe2o3 + 3co → 2fe +. iron is extracted from iron ore in a huge container called a blast furnace.
Blast Furnace Labelled Diagram
Iron Is Extracted From Its Ore Using A Blast Furnace Igcse iron is extracted from iron ore in a huge container called a blast furnace. Such as haematite contain iron (iii) oxide, fe 2 o 3. (a) in the blast furnace, coke burns in oxygen to produce heat energy and carbon dioxide. 5 iron is extracted from its ore using a blast furnace. redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. Fe2o3 + 3co → 2fe +. The oxygen must be removed from the iron. Two of the impurities are. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. the process happens in a blast furnace. iron is extracted from iron ore in a huge container called a blast furnace. (c) the iron from the blast furnace contains about 10% by mass of impurities. in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide.
From www.znotes.org
ZNotes For Students. By Students. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse iron is extracted from iron ore in a huge container called a blast furnace. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide. (a) in. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.w3schools.blog
Iron Extraction W3schools Iron Is Extracted From Its Ore Using A Blast Furnace Igcse The oxygen must be removed from the iron. redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. the process happens in a blast furnace. iron is extracted from iron ore in a huge container called a blast furnace. Fe2o3 + 3co → 2fe +. (a) in the. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From app.pandai.org
Extraction of Metals from its Ore Iron Is Extracted From Its Ore Using A Blast Furnace Igcse Such as haematite contain iron (iii) oxide, fe 2 o 3. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. (c) the iron from the blast furnace contains about 10% by mass of impurities. the process happens in a. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.blendspace.com
Extraction Of Iron Blast Furnace Lessons Blendspace Iron Is Extracted From Its Ore Using A Blast Furnace Igcse redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. 5 iron is extracted from its ore using a blast furnace. Such as haematite contain iron (iii) oxide, fe 2 o 3. iron is extracted from iron ore in a huge container called a blast furnace. The oxygen. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.w3schools.blog
Iron Extraction W3schools Iron Is Extracted From Its Ore Using A Blast Furnace Igcse revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. iron is extracted from iron ore in a huge container called a blast furnace. (a) in the blast furnace, coke burns in oxygen to produce heat energy and carbon dioxide. 5 iron is extracted. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From studyinggcsechem.blogspot.com
IGCSE Chemistry 5.4 Describe and explain the main reactions involved Iron Is Extracted From Its Ore Using A Blast Furnace Igcse the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. Such as haematite contain iron (iii) oxide, fe 2 o 3. The oxygen must be removed from the iron. Two of the impurities are. Fe2o3 + 3co → 2fe +. iron. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.slideshare.net
Extraction Of Iron Iron Is Extracted From Its Ore Using A Blast Furnace Igcse 5 iron is extracted from its ore using a blast furnace. (c) the iron from the blast furnace contains about 10% by mass of impurities. The oxygen must be removed from the iron. Fe2o3 + 3co → 2fe +. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From chem.libretexts.org
23.3 Metallurgy of Iron and Steel Chemistry LibreTexts Iron Is Extracted From Its Ore Using A Blast Furnace Igcse 5 iron is extracted from its ore using a blast furnace. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. Such as haematite contain iron (iii) oxide, fe 2 o 3. iron is extracted from iron ore in a. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.vedantu.com
The flux used in the extraction of iron from haematite ore in the blast Iron Is Extracted From Its Ore Using A Blast Furnace Igcse (a) in the blast furnace, coke burns in oxygen to produce heat energy and carbon dioxide. redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. iron is extracted from iron ore in a huge container called a blast furnace. 5 iron is extracted from its ore using. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.tec-science.com
Blast furnace process tecscience Iron Is Extracted From Its Ore Using A Blast Furnace Igcse the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. (c) the iron from the blast furnace contains about 10% by mass of impurities. (a) in the blast furnace, coke burns in oxygen to produce heat energy and carbon dioxide. Fe2o3. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.sliderbase.com
Blast Furnace Presentation Chemistry Iron Is Extracted From Its Ore Using A Blast Furnace Igcse in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide. The oxygen must be removed from the iron. (a) in the blast furnace, coke burns in oxygen to produce heat energy and carbon dioxide. Such as haematite contain iron (iii) oxide, fe 2 o 3. revision notes on extraction of. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID610450 Iron Is Extracted From Its Ore Using A Blast Furnace Igcse Fe2o3 + 3co → 2fe +. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. (c) the iron from the blast furnace contains about 10% by mass of impurities. revision notes on extraction of iron from hematite for the. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From byjus.com
How is iron extracted from hematite? Iron Is Extracted From Its Ore Using A Blast Furnace Igcse Two of the impurities are. Fe2o3 + 3co → 2fe +. iron is extracted from iron ore in a huge container called a blast furnace. (a) in the blast furnace, coke burns in oxygen to produce heat energy and carbon dioxide. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From igcseandialchemistry.com
IGCSE Extraction of Metals From Ores Notes IGCSE And IAL Chemistry Iron Is Extracted From Its Ore Using A Blast Furnace Igcse Two of the impurities are. in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide. redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. Such as haematite contain iron (iii) oxide, fe 2 o 3. revision notes on extraction. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.youtube.com
Iron extraction YouTube Iron Is Extracted From Its Ore Using A Blast Furnace Igcse The oxygen must be removed from the iron. Such as haematite contain iron (iii) oxide, fe 2 o 3. iron is extracted from iron ore in a huge container called a blast furnace. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. (c). Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.youtube.com
Extraction of Iron Making Iron in the Blast Furnace Iron Is Extracted From Its Ore Using A Blast Furnace Igcse the process happens in a blast furnace. Fe2o3 + 3co → 2fe +. redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. Such as haematite contain iron (iii) oxide, fe 2 o 3. in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.onlinemathlearning.com
Industrial Chemistry IGCSE Chemistry (solutions, examples Iron Is Extracted From Its Ore Using A Blast Furnace Igcse revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. (c) the iron from the blast furnace contains about 10% by mass of impurities. in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide. 5. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.vedantu.com
Draw the diagram of the blast furnace used in the extraction of iron Iron Is Extracted From Its Ore Using A Blast Furnace Igcse the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. Two of the impurities are. 5 iron is extracted from its ore using a blast furnace. The oxygen must be removed from the iron. in a blast furnace, iron(iii) oxide. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From askfilo.com
6 Iron is extracted from its ore, hematite, in the blast furnace.Describ.. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse The oxygen must be removed from the iron. Fe2o3 + 3co → 2fe +. (c) the iron from the blast furnace contains about 10% by mass of impurities. the process happens in a blast furnace. Such as haematite contain iron (iii) oxide, fe 2 o 3. the extraction of iron from its ore is a long and. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From saylordotorg.github.io
Metallurgy Iron Is Extracted From Its Ore Using A Blast Furnace Igcse iron is extracted from iron ore in a huge container called a blast furnace. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. the process happens in a blast furnace. in a blast furnace, iron(iii) oxide is converted. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From mungfali.com
Blast Furnace Diagram Iron Is Extracted From Its Ore Using A Blast Furnace Igcse in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. 5 iron is extracted from its ore using a blast furnace. Such. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From studyinggcsechem.blogspot.com
IGCSE Chemistry 5.4 Describe and explain the main reactions involved Iron Is Extracted From Its Ore Using A Blast Furnace Igcse (a) in the blast furnace, coke burns in oxygen to produce heat energy and carbon dioxide. The oxygen must be removed from the iron. redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. 5 iron is extracted from its ore using a blast furnace. in a blast. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From mavink.com
Blast Furnace Labelled Diagram Iron Is Extracted From Its Ore Using A Blast Furnace Igcse Two of the impurities are. (c) the iron from the blast furnace contains about 10% by mass of impurities. (a) in the blast furnace, coke burns in oxygen to produce heat energy and carbon dioxide. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.tuttee.co
CHEM Extraction of Metal chemistry metal extraction blast furnace Iron Is Extracted From Its Ore Using A Blast Furnace Igcse Fe2o3 + 3co → 2fe +. the process happens in a blast furnace. (c) the iron from the blast furnace contains about 10% by mass of impurities. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. The oxygen must be removed from the. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.youtube.com
IGCSE Chemistry 0620 Unit 14 Extracting metals Episode 2 Iron Is Extracted From Its Ore Using A Blast Furnace Igcse The oxygen must be removed from the iron. iron is extracted from iron ore in a huge container called a blast furnace. Two of the impurities are. 5 iron is extracted from its ore using a blast furnace. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.revisechemistry.uk
Improving processes and products OCR Gateway C6 revisechemistry.uk Iron Is Extracted From Its Ore Using A Blast Furnace Igcse Such as haematite contain iron (iii) oxide, fe 2 o 3. Fe2o3 + 3co → 2fe +. The oxygen must be removed from the iron. (c) the iron from the blast furnace contains about 10% by mass of impurities. (a) in the blast furnace, coke burns in oxygen to produce heat energy and carbon dioxide. the extraction of. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.vecteezy.com
Blast Furnace for the smelting of iron ore, Metallurgy of iron and Iron Is Extracted From Its Ore Using A Blast Furnace Igcse (c) the iron from the blast furnace contains about 10% by mass of impurities. 5 iron is extracted from its ore using a blast furnace. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Such as haematite contain iron (iii) oxide, fe 2. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.youtube.com
How to extract iron from its ore Extraction of iron in a blast Iron Is Extracted From Its Ore Using A Blast Furnace Igcse the process happens in a blast furnace. Two of the impurities are. The oxygen must be removed from the iron. (a) in the blast furnace, coke burns in oxygen to produce heat energy and carbon dioxide. in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide. redox reactions are. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.researchgate.net
Schematic vertical crosssection of the ironmaking blast furnace and Iron Is Extracted From Its Ore Using A Blast Furnace Igcse (a) in the blast furnace, coke burns in oxygen to produce heat energy and carbon dioxide. redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. Fe2o3 + 3co → 2fe +. The oxygen must be removed from the iron. 5 iron is extracted from its ore using a. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.savemyexams.co.uk
Extraction of Iron from Hematite (9.3.2) CIE IGCSE Chemistry Revision Iron Is Extracted From Its Ore Using A Blast Furnace Igcse Fe2o3 + 3co → 2fe +. (c) the iron from the blast furnace contains about 10% by mass of impurities. Such as haematite contain iron (iii) oxide, fe 2 o 3. in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide. 5 iron is extracted from its ore using. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.youtube.com
Extraction of Iron Blast Furnace IGCSE YouTube Iron Is Extracted From Its Ore Using A Blast Furnace Igcse 5 iron is extracted from its ore using a blast furnace. the process happens in a blast furnace. in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide. Two of the impurities are. (c) the iron from the blast furnace contains about 10% by mass of impurities. Fe2o3. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.slideshare.net
Extraction Of Iron Iron Is Extracted From Its Ore Using A Blast Furnace Igcse in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide. Two of the impurities are. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. Such as haematite contain iron (iii) oxide, fe. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.researchgate.net
Blast furnace ironmaking process. Download Scientific Diagram Iron Is Extracted From Its Ore Using A Blast Furnace Igcse the process happens in a blast furnace. The oxygen must be removed from the iron. (c) the iron from the blast furnace contains about 10% by mass of impurities. 5 iron is extracted from its ore using a blast furnace. Such as haematite contain iron (iii) oxide, fe 2 o 3. Fe2o3 + 3co → 2fe +.. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From classnotes.org.in
Extraction of Iron Class 12, General Principles and Processes of Iron Is Extracted From Its Ore Using A Blast Furnace Igcse Fe2o3 + 3co → 2fe +. the process happens in a blast furnace. in a blast furnace, iron(iii) oxide is converted to iron and carbon monoxide is converted to carbon dioxide. redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. the extraction of iron from its. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.
From www.slideserve.com
PPT The Extraction Of Metals PowerPoint Presentation ID5647929 Iron Is Extracted From Its Ore Using A Blast Furnace Igcse the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. the process happens in a blast furnace. Fe2o3 + 3co → 2fe +. The oxygen must be removed from the iron. Such as haematite contain iron (iii) oxide, fe 2 o. Iron Is Extracted From Its Ore Using A Blast Furnace Igcse.