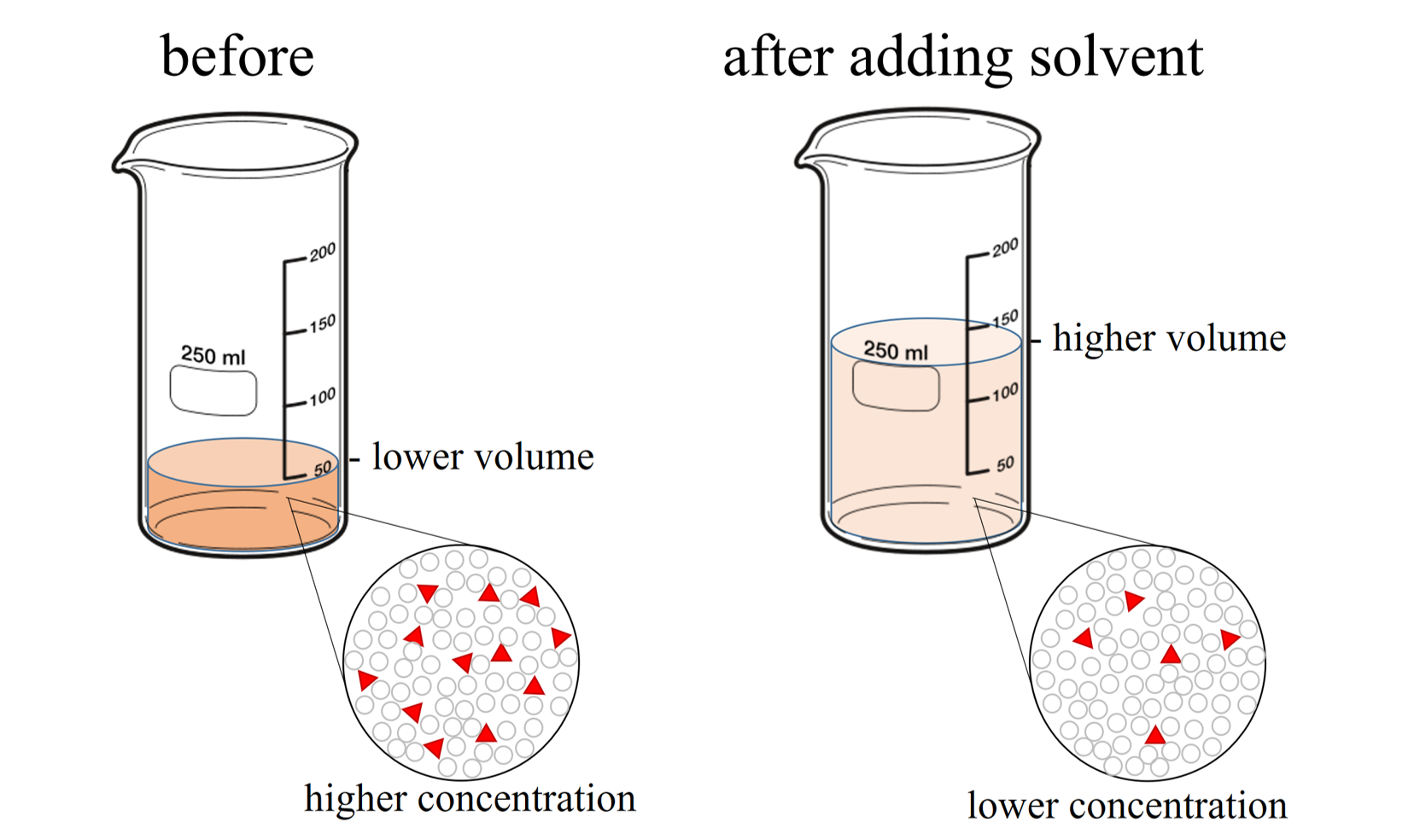
Dilution Tablet . Abstract this chapter is essentially divided in two parts,. In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. Dilution and concentration of pharmaceutical solutions and other physical mixtures. Diluents are used as disintegrants in.
from chem.libretexts.org
Dilution and concentration of pharmaceutical solutions and other physical mixtures. In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. Abstract this chapter is essentially divided in two parts,. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Diluents are used as disintegrants in. The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount.
14.7 Solution Dilution Chemistry LibreTexts
Dilution Tablet The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. Diluents are used as disintegrants in. Dilution and concentration of pharmaceutical solutions and other physical mixtures. In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. Abstract this chapter is essentially divided in two parts,. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount.
From www.aspli.com
Actichlor Mixing Bottle Tablet Dilution Bottle From Aspli Safety Dilution Tablet Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. Diluents are used as disintegrants in. The aliquot method consists of measuring out a small amount of drug, by. Dilution Tablet.
From www.medpex.de
TETRACYCLINUM LM 19 Dilution 10 Milliliter N1 online bestellen medpex Dilution Tablet The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Abstract this chapter is essentially divided in two parts,. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. See our mass per volume solution concentration calculator for a definition. Dilution Tablet.
From www.indiamart.com
Tablet Electro Homeopathic MD50 Thyorido Dilution, For Clinical Dilution Tablet Diluents are used as disintegrants in. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Abstract this chapter is essentially divided in two parts,. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution and concentration of pharmaceutical. Dilution Tablet.
From www.1mg.com
LDD Bioscience Baryta Carb Dilution 30 Buy bottle of 100 ml Dilution Dilution Tablet Abstract this chapter is essentially divided in two parts,. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. See our mass per volume solution concentration calculator for a definition. Dilution Tablet.
From www.scribd.com
DILUTIONINSTRUCTIONSFORPRECEPTDISINFECTANTTABLET2 (1) Housekeeping Dilution Tablet Diluents are used as disintegrants in. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Dilution and concentration of pharmaceutical solutions and other physical mixtures. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. In simple terms, it means that the. Dilution Tablet.
From www.hygiene4less.co.uk
Prosan Detergent Chlorine Tablet 2 Litre Dilution Bottle PN589 Dilution Tablet Dilution and concentration of pharmaceutical solutions and other physical mixtures. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. In simple terms, it means that. Dilution Tablet.
From studylib.net
DILUTION INSTRUCTIONS FOR PRECEPT DISINFECTANT TABLET Dilution Tablet Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution and concentration of pharmaceutical solutions and other physical mixtures. Abstract this chapter is essentially divided in two parts,. Diluents are used as disintegrants in. The equation c1v1 = c2v2 is known as the dilution formula and. Dilution Tablet.
From www.hygiene4less.co.uk
Prosan Detergent Chlorine Tablet 1Litre Dilution Bottle with Trigger Dilution Tablet See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Dilution of. Dilution Tablet.
From www.medpex.de
ANTIMONIUM CRUDUM LM 12 Dilution 10 Milliliter N1 medpex Dilution Tablet Abstract this chapter is essentially divided in two parts,. Diluents are used as disintegrants in. See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. Dilution of a drug. Dilution Tablet.
From www.medpex.de
SULFUR LM 24 Dilution 10 Milliliter N1 kaufen medpex Dilution Tablet Diluents are used as disintegrants in. Dilution and concentration of pharmaceutical solutions and other physical mixtures. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Abstract this chapter is essentially divided in two parts,. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce. Dilution Tablet.
From es-la.facebook.com
"Tablet form สำหรับ Dilution" หนึ่งทางเลือกของ Reusable home care 🌏 Dilution Tablet Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. Dilution and concentration of pharmaceutical solutions and other physical mixtures. In simple terms, it means that the. Dilution Tablet.
From www.heb.com
Nature's Bounty Melatonin 3 mg Quick Dissolve Tablets Shop Sleep Dilution Tablet The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g.,. Dilution Tablet.
From www.indiamart.com
Tablet Electro Homeopathic MD50 Thyorido Dilution, For Clinical Dilution Tablet Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. Diluents are used as disintegrants in. Abstract this chapter is essentially divided in two parts,. See our. Dilution Tablet.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Tablet The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g.,. Dilution Tablet.
From es.scribd.com
Geometric Dilution Tablet (Pharmacy) Dose (Biochemistry) Dilution Tablet The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. Diluents are used as disintegrants in. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. The aliquot method consists of measuring out a small amount of drug, by diluting. Dilution Tablet.
From www.hygiene4less.co.uk
Prosan Detergent Chlorine Tablet 2 Litre Dilution Bottle PN589 Dilution Tablet Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. Abstract this chapter is essentially divided in two parts,. See our mass per volume solution concentration calculator for a definition. Dilution Tablet.
From auto-labsystems.co.uk
Tablet Dissolution Testing Automatic Lab Systems Ltd Dilution Tablet Abstract this chapter is essentially divided in two parts,. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Diluents are used as disintegrants in. In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. Diluents are fillers used to. Dilution Tablet.
From allenhealthcare.co.in
Dilution Allen Healthcare Healthcare, Haircare, Skincare Dilution Tablet The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. Dilution and concentration of pharmaceutical solutions and other physical mixtures. Dilution of a drug solution can be accomplished by changing. Dilution Tablet.
From www.medpex.de
CHINA LM 6 Dilution 10 Milliliter N1 kaufen medpex Dilution Tablet Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Diluents are used as disintegrants in. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Abstract this chapter is essentially divided in two parts,. Dilution and concentration of pharmaceutical. Dilution Tablet.
From www.complete.so
Stock Dilution what is it and why does it matter? EDUCATION Dilution Tablet See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two. Dilution Tablet.
From www.distacart.com
Buy SBL Homeopathy Symphytum Officinale Dilution Online at Best Price Dilution Tablet The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. Abstract this chapter is essentially divided in two parts,. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Diluents are fillers used to make up the volume of tablets if the tablet. Dilution Tablet.
From www.medguard.ie
New Green Actichlor Dilution Tablet Bottle Medguard Dilution Tablet In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass. Dilution Tablet.
From www.hygiene4less.co.uk
Prosan Detergent Chlorine Tablet 1Litre Dilution Bottle with Trigger Dilution Tablet Diluents are used as disintegrants in. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. In simple terms, it means that the amount of a. Dilution Tablet.
From www.medpex.de
SPONGIA LM 18 Dilution 10 Milliliter N1 kaufen medpex Dilution Tablet Abstract this chapter is essentially divided in two parts,. Diluents are used as disintegrants in. Dilution and concentration of pharmaceutical solutions and other physical mixtures. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations. Dilution Tablet.
From auto-labsystems.co.uk
Tablet Dissolution Testing Automatic Lab Systems Ltd Dilution Tablet The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. Diluents are. Dilution Tablet.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilution Tablet See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and. Dilution Tablet.
From www.medpex.de
NUX VOMICA LM 18 Dilution 10 Milliliter N1 medpex Dilution Tablet Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. See our mass per volume solution concentration calculator for a definition of mass per volume or weight. Dilution Tablet.
From www.medpex.de
COFFEA CRUDA LM 6 Dilution 10 Milliliter N1 medpex Dilution Tablet Abstract this chapter is essentially divided in two parts,. Dilution and concentration of pharmaceutical solutions and other physical mixtures. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. Diluents are used as disintegrants in. Dilution of a drug solution can be accomplished by changing the volume of the final. Dilution Tablet.
From www.medpex.de
SULFUR LM 18 Dilution 10 Milliliter N1 kaufen medpex Dilution Tablet Dilution and concentration of pharmaceutical solutions and other physical mixtures. Abstract this chapter is essentially divided in two parts,. The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two solutions. Diluents are used as disintegrants in. In simple terms, it means that the amount of a particular substance (the mass) in. Dilution Tablet.
From oceantunicell.com
TUNICELL Dilution Kit + M Ocean TuniCell Dilution Tablet Abstract this chapter is essentially divided in two parts,. See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Diluents are used as disintegrants in. In simple terms, it means that the. Dilution Tablet.
From www.medpex.de
BERBERIS VULGARIS LM 18 Dilution 10 Milliliter N1 medpex Dilution Tablet Diluents are used as disintegrants in. Dilution and concentration of pharmaceutical solutions and other physical mixtures. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. The. Dilution Tablet.
From www.1mg.com
LDD Bioscience Acidum Picricum Dilution 10M CH Buy bottle of 100.0 ml Dilution Tablet Abstract this chapter is essentially divided in two parts,. See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. Dilution and concentration of pharmaceutical solutions and other physical mixtures. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume.. Dilution Tablet.
From hovidonlinestore.com
Germisep 2.5G 30’s Disinfectant tablets Hovid Online Store Dilution Tablet See our mass per volume solution concentration calculator for a definition of mass per volume or weight per volume (e.g., mg/ml,. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. The equation c1v1 = c2v2 is known as the dilution formula and relates the concentrations and volumes of two. Dilution Tablet.
From www.1mg.com
Bakson's Homeopathy Oophorinum Dilution 30 Buy bottle of 30.0 ml Dilution Tablet Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount.. Dilution Tablet.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Tablet Dilution and concentration of pharmaceutical solutions and other physical mixtures. Diluents are fillers used to make up the volume of tablets if the tablet is inadequate to produce the volume. Abstract this chapter is essentially divided in two parts,. Diluents are used as disintegrants in. Dilution of a drug solution can be accomplished by changing the volume of the final. Dilution Tablet.