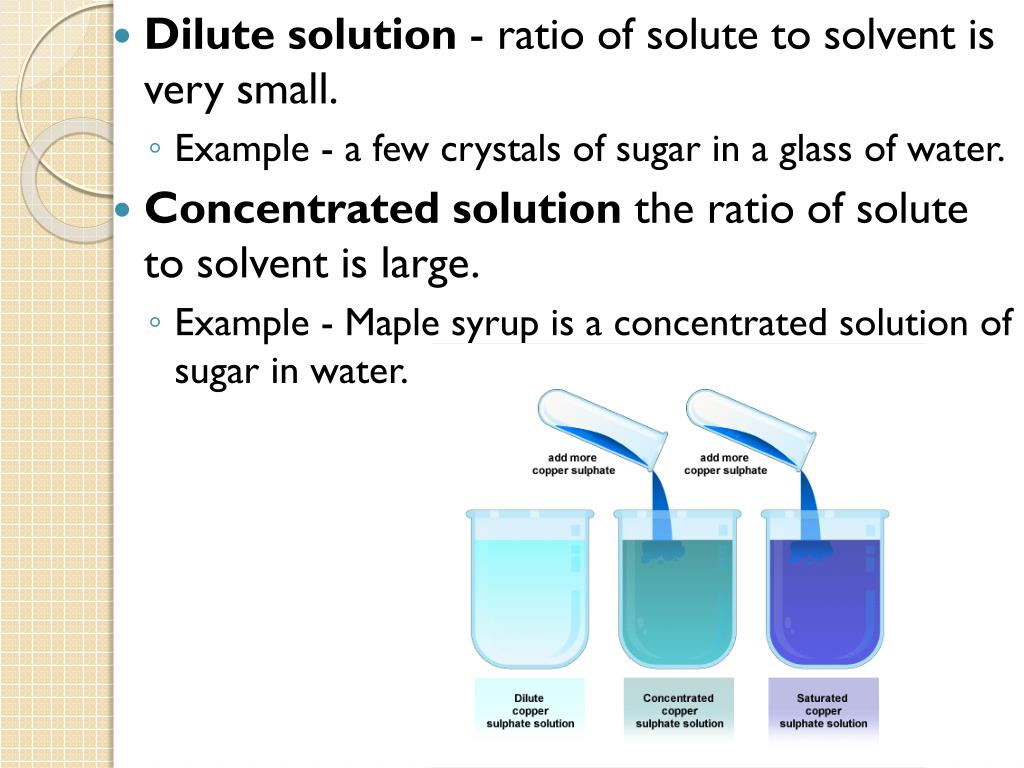
Dilute Solution Example At Home . Dilution is the addition of. We are often concerned with how much solute is dissolved in a given amount of. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Understand how stock solutions are used in the laboratory. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. For example, 10.0 µl of a 1.76 m solution of hno 3 (nitric acid) are diluted into 10.0 ml of distilled water; Learn how to dilute and concentrate solutions. For example, if you have a glass. For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.
from www.slideserve.com
We are often concerned with how much solute is dissolved in a given amount of. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Dilution is the addition of. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. For example, if you have a glass. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Understand how stock solutions are used in the laboratory. For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.
PPT Solutions and Concentration (p123 136 ) PowerPoint Presentation
Dilute Solution Example At Home Often, a worker will need to change the concentration of a solution by changing the amount of solvent. We are often concerned with how much solute is dissolved in a given amount of. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate solutions. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. For example, if you have a glass. Understand how stock solutions are used in the laboratory. Dilution is the addition of. For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. For example, 10.0 µl of a 1.76 m solution of hno 3 (nitric acid) are diluted into 10.0 ml of distilled water;
From www.slideserve.com
PPT Chapter 15 Solutions PowerPoint Presentation, free download ID Dilute Solution Example At Home This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. We are often concerned with how much solute is dissolved in a given amount. Dilute Solution Example At Home.
From studylib.net
Dilutions Dilute Solution Example At Home Understand how stock solutions are used in the laboratory. Learn how to dilute and concentrate solutions. For example, if you have a glass. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. We are often concerned with how much solute is dissolved in a given amount. Dilute Solution Example At Home.
From www.chegg.com
Solved Match the dilution with the correct solution shown in Dilute Solution Example At Home Understand how stock solutions are used in the laboratory. Learn how to dilute and concentrate solutions. For example, 10.0 µl of a 1.76 m solution of hno 3 (nitric acid) are diluted into 10.0 ml of distilled water; A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. Dilute Solution Example At Home.
From www.slideserve.com
PPT Solutions and Concentration (p123 136 ) PowerPoint Presentation Dilute Solution Example At Home A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Learn how to dilute and concentrate solutions. For example, 10.0 µl of a 1.76 m solution of hno 3 (nitric acid) are diluted into 10.0 ml of distilled water; Understand how stock solutions are used in the. Dilute Solution Example At Home.
From studylib.net
Solutions and Dilutions Dilute Solution Example At Home For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. We are. Dilute Solution Example At Home.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilute Solution Example At Home Dilution is the addition of. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Often, a worker will need to change the concentration. Dilute Solution Example At Home.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilute Solution Example At Home For example, 10.0 µl of a 1.76 m solution of hno 3 (nitric acid) are diluted into 10.0 ml of distilled water; A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution is the process of making a solution or scientific sample less concentrated by adding. Dilute Solution Example At Home.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilute Solution Example At Home Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. We are often concerned with how much solute is dissolved in a given amount. Dilute Solution Example At Home.
From www.slideserve.com
PPT Dilute Liquid Solutions SVNA 10.4,12.1 PowerPoint Presentation Dilute Solution Example At Home Understand how stock solutions are used in the laboratory. We are often concerned with how much solute is dissolved in a given amount of. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Often, a worker will need to change the concentration of a solution by. Dilute Solution Example At Home.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilute Solution Example At Home For example, if you have a glass. Dilution is the addition of. For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water. Learn how to dilute and concentrate solutions. Dilution is the process of making. Dilute Solution Example At Home.
From www.slideserve.com
PPT Solutions Part II PowerPoint Presentation, free download ID4272422 Dilute Solution Example At Home Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water.. Dilute Solution Example At Home.
From slidetodoc.com
WATER AND SOLUTIONS A solution is a mixture Dilute Solution Example At Home Understand how stock solutions are used in the laboratory. Dilution is the addition of. For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water. For example, if you have a glass. We are often concerned. Dilute Solution Example At Home.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution Example At Home Understand how stock solutions are used in the laboratory. For example, 10.0 µl of a 1.76 m solution of hno 3 (nitric acid) are diluted into 10.0 ml of distilled water; Often, a worker will need to change the concentration of a solution by changing the amount of solvent. For example, if you have a glass. Dilution is the addition. Dilute Solution Example At Home.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Solution Example At Home For example, if you have a glass. Dilution is the addition of. Learn how to dilute and concentrate solutions. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. For example, 10.0 µl of a 1.76 m solution of hno 3 (nitric acid) are diluted into 10.0. Dilute Solution Example At Home.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID Dilute Solution Example At Home Understand how stock solutions are used in the laboratory. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. We are often concerned with. Dilute Solution Example At Home.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilute Solution Example At Home Dilution is the addition of. For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water. For example, if you have a glass. A dilution is a process where the concentration of a solution is lowered. Dilute Solution Example At Home.
From www.haikudeck.com
Mixtures And Solutions by Alexandra Stearns Dilute Solution Example At Home Understand how stock solutions are used in the laboratory. Dilution is the addition of. For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water. Dilution is the process of making a solution or scientific sample. Dilute Solution Example At Home.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Dilute Solution Example At Home We are often concerned with how much solute is dissolved in a given amount of. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Dilution is the addition of.. Dilute Solution Example At Home.
From www.youtube.com
Aqueous, Dilute And Concentrated Solution, General Science Lecture Dilute Solution Example At Home Often, a worker will need to change the concentration of a solution by changing the amount of solvent. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. We are often concerned with how much solute is dissolved in a given amount of. For example, if you. Dilute Solution Example At Home.
From www.teachoo.com
Difference between Saturated and Unsaturated Solution Teachoo Dilute Solution Example At Home This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding. Dilute Solution Example At Home.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Dilute Solution Example At Home We are often concerned with how much solute is dissolved in a given amount of. Learn how to dilute and concentrate solutions. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Understand how stock solutions are used in the laboratory. This guide will describe the process. Dilute Solution Example At Home.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution Example At Home We are often concerned with how much solute is dissolved in a given amount of. Learn how to dilute and concentrate solutions. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. A dilution is a process where the concentration of a solution is lowered by adding. Dilute Solution Example At Home.
From www.slideserve.com
PPT Introduction to solutions PowerPoint Presentation, free download Dilute Solution Example At Home Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water. We are often concerned with how much solute. Dilute Solution Example At Home.
From www.youtube.com
Dilute and Concentrated Solution YouTube Dilute Solution Example At Home This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Learn how to dilute and concentrate solutions. We are often concerned with how much. Dilute Solution Example At Home.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilute Solution Example At Home For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water. For example, 10.0 µl of a 1.76 m solution of hno 3 (nitric acid) are diluted into 10.0 ml of distilled water; Often, a worker. Dilute Solution Example At Home.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilute Solution Example At Home Understand how stock solutions are used in the laboratory. Learn how to dilute and concentrate solutions. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. For example, if you have a glass. For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter. Dilute Solution Example At Home.
From www.omo.com
Dilute At Home Refill Omo Dilute Solution Example At Home Often, a worker will need to change the concentration of a solution by changing the amount of solvent. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Learn how to dilute and concentrate solutions. Dilution is the addition of. Understand how stock solutions are used in. Dilute Solution Example At Home.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilute Solution Example At Home Dilution is the addition of. For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for. Dilute Solution Example At Home.
From www.dailymotion.com
What is Dilute Solution _ Examples of Dilute Solution _ Chemistry Dilute Solution Example At Home A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for. Dilute Solution Example At Home.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilute Solution Example At Home Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. For example, if you have a glass. A dilution is a process where the concentration of a solution is lowered by adding solvent to the. Dilute Solution Example At Home.
From ar.inspiredpencil.com
Dilute Solution Diagram Dilute Solution Example At Home Understand how stock solutions are used in the laboratory. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Learn how to dilute and concentrate solutions. This guide will describe. Dilute Solution Example At Home.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Dilute Solution Example At Home For instance, hydrogen peroxide (h 2 o 2 ) as sold over the counter in drug stores, to be used in homes as a disinfectant of bleaching agent, is a dilute 3% solution in water. Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount. Dilute Solution Example At Home.
From ar.inspiredpencil.com
Dilute Solution Diagram Dilute Solution Example At Home Dilution is the addition of. Learn how to dilute and concentrate solutions. For example, if you have a glass. For example, 10.0 µl of a 1.76 m solution of hno 3 (nitric acid) are diluted into 10.0 ml of distilled water; Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Understand how. Dilute Solution Example At Home.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Solution Example At Home For example, 10.0 µl of a 1.76 m solution of hno 3 (nitric acid) are diluted into 10.0 ml of distilled water; We are often concerned with how much solute is dissolved in a given amount of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. For example, if you have. Dilute Solution Example At Home.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilute Solution Example At Home For example, 10.0 µl of a 1.76 m solution of hno 3 (nitric acid) are diluted into 10.0 ml of distilled water; This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Understand how stock solutions are used in the laboratory. Dilution is the addition of. Learn. Dilute Solution Example At Home.