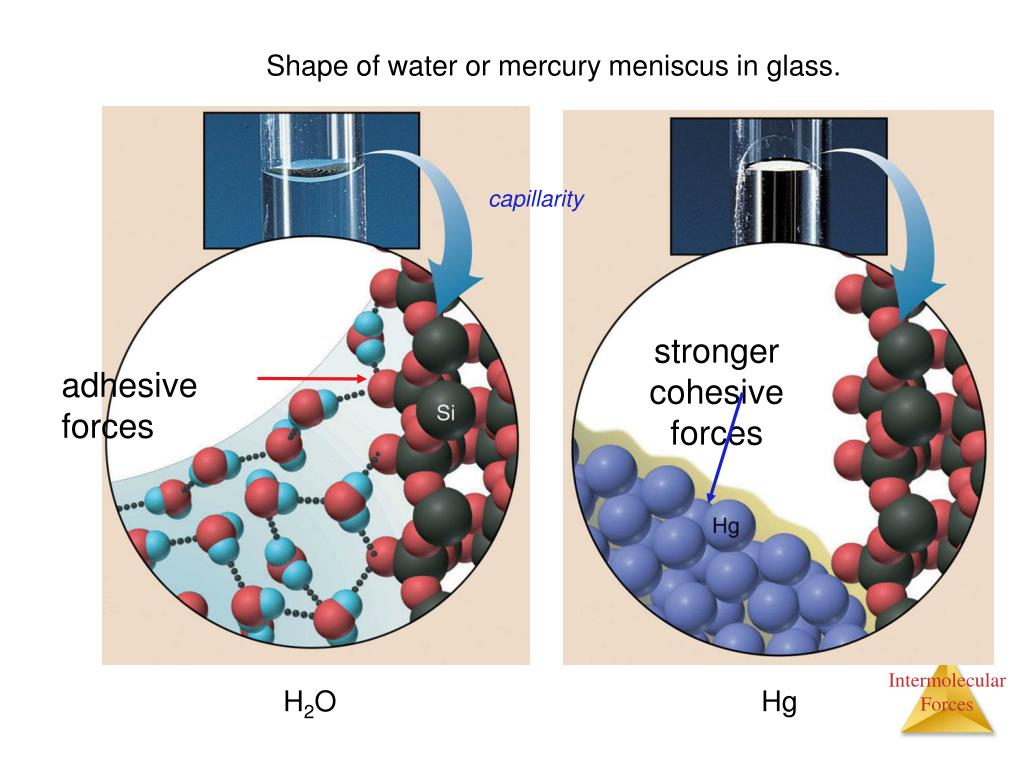
Why Does Surface Tension Increase With Intermolecular Forces . Surface tension the phenomenon that maintains the surface interface between liquid and gas. It allows you to place needles and insects on. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This property is caused by intermolecular forces, which keep the molecules. Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces.
from www.slideserve.com
Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. This property is caused by intermolecular forces, which keep the molecules. It allows you to place needles and insects on. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary. Surface tension the phenomenon that maintains the surface interface between liquid and gas. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop.
PPT Chapter 9 Intermolecular Forces, Liquids, and Solids PowerPoint
Why Does Surface Tension Increase With Intermolecular Forces It allows you to place needles and insects on. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. It allows you to place needles and insects on. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. This property is caused by intermolecular forces, which keep the molecules. Surface tension the phenomenon that maintains the surface interface between liquid and gas. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
From www.slideserve.com
PPT Chapter 12 Intermolecular Forces PowerPoint Presentation, free Why Does Surface Tension Increase With Intermolecular Forces This property is caused by intermolecular forces, which keep the molecules. Since these intermolecular forces vary. It allows you to place needles and insects on. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. When your finger is pricked, a drop of blood forms and holds together due. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download Why Does Surface Tension Increase With Intermolecular Forces Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Since these intermolecular forces vary. This property is caused by intermolecular. Why Does Surface Tension Increase With Intermolecular Forces.
From jsmithmoore.com
Intermolecular forces Why Does Surface Tension Increase With Intermolecular Forces Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. When your finger is pricked, a drop of blood forms and holds together due to surface. Why Does Surface Tension Increase With Intermolecular Forces.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity Why Does Surface Tension Increase With Intermolecular Forces Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. This property is caused by intermolecular forces, which keep the molecules. Surface tension the phenomenon that maintains the surface interface between liquid and gas. Surface tension is the energy, or work, required to increase the surface area of a. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Does Surface Tension Increase With Intermolecular Forces Since these intermolecular forces vary. It allows you to place needles and insects on. Surface tension the phenomenon that maintains the surface interface between liquid and gas. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension, the amount of energy required to increase the surface area of. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Implication of Intermolecular Forces PowerPoint Presentation Why Does Surface Tension Increase With Intermolecular Forces It allows you to place needles and insects on. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension the phenomenon that maintains the surface interface between liquid and gas. Surface tension is the energy required to increase the surface area of a liquid by a unit amount. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint Why Does Surface Tension Increase With Intermolecular Forces Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Surface tension, the amount of energy required to increase the. Why Does Surface Tension Increase With Intermolecular Forces.
From irajlorenzeno.blob.core.windows.net
Does Surface Tension Increase With Intermolecular Forces at Why Does Surface Tension Increase With Intermolecular Forces When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. It allows you to place needles and insects on. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. Surface tension the phenomenon. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of Why Does Surface Tension Increase With Intermolecular Forces Surface tension the phenomenon that maintains the surface interface between liquid and gas. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. It allows you to place needles and insects on. Surface tension, the amount of energy required to increase the surface area. Why Does Surface Tension Increase With Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download Why Does Surface Tension Increase With Intermolecular Forces Surface tension the phenomenon that maintains the surface interface between liquid and gas. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape.. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Polarity and Intermolecular Forces PowerPoint Presentation, free Why Does Surface Tension Increase With Intermolecular Forces Surface tension the phenomenon that maintains the surface interface between liquid and gas. Since these intermolecular forces vary. This property is caused by intermolecular forces, which keep the molecules. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to increase the surface area. Why Does Surface Tension Increase With Intermolecular Forces.
From www.researchgate.net
2. Intermolecular forces causing the surface tension phenomena (A) and Why Does Surface Tension Increase With Intermolecular Forces Surface tension the phenomenon that maintains the surface interface between liquid and gas. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. It allows you to place needles and insects on. Surface tension is the elastic property of the surface of a liquid, such as. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint Why Does Surface Tension Increase With Intermolecular Forces When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Surface tension the phenomenon that maintains the surface interface between liquid and gas. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape.. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint Why Does Surface Tension Increase With Intermolecular Forces Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Since these intermolecular forces vary. Surface tension the phenomenon that maintains. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint Why Does Surface Tension Increase With Intermolecular Forces Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. This property is caused by intermolecular forces, which keep the molecules. Surface tension the. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint Why Does Surface Tension Increase With Intermolecular Forces This property is caused by intermolecular forces, which keep the molecules. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. It allows you to place needles. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint Why Does Surface Tension Increase With Intermolecular Forces When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. It allows you to place needles and insects on. Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. Surface tension. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Why Does Surface Tension Increase With Intermolecular Forces Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Surface tension is the energy required to increase the surface area of a. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular forces Generalizing properties PowerPoint Why Does Surface Tension Increase With Intermolecular Forces Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary. Surface tension the phenomenon that maintains the surface interface between liquid and gas. This property is caused by intermolecular forces, which keep the molecules. When your finger is pricked, a drop of blood forms and. Why Does Surface Tension Increase With Intermolecular Forces.
From chem.libretexts.org
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and Why Does Surface Tension Increase With Intermolecular Forces Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Surface tension the phenomenon that maintains the surface interface between liquid and gas.. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of Why Does Surface Tension Increase With Intermolecular Forces Surface tension the phenomenon that maintains the surface interface between liquid and gas. Since these intermolecular forces vary. It allows you to place needles and insects on. Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. Surface tension is the energy required to increase the surface. Why Does Surface Tension Increase With Intermolecular Forces.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Why Does Surface Tension Increase With Intermolecular Forces This property is caused by intermolecular forces, which keep the molecules. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces.. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids and Solids PowerPoint Presentation Why Does Surface Tension Increase With Intermolecular Forces Surface tension the phenomenon that maintains the surface interface between liquid and gas. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Chapter 9 Intermolecular Forces, Liquids, and Solids PowerPoint Why Does Surface Tension Increase With Intermolecular Forces Surface tension the phenomenon that maintains the surface interface between liquid and gas. Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to.. Why Does Surface Tension Increase With Intermolecular Forces.
From www.youtube.com
Surface tension and capillary action Intermolecular Forces meriSTEM Why Does Surface Tension Increase With Intermolecular Forces Since these intermolecular forces vary. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Surface tension is the elastic. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint Why Does Surface Tension Increase With Intermolecular Forces Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Surface tension the phenomenon that maintains the surface interface between liquid and gas.. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint Why Does Surface Tension Increase With Intermolecular Forces Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Surface tension the phenomenon that maintains the surface interface between liquid and gas. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to. Why Does Surface Tension Increase With Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download Why Does Surface Tension Increase With Intermolecular Forces It allows you to place needles and insects on. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. Since these intermolecular forces vary. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. This. Why Does Surface Tension Increase With Intermolecular Forces.
From www.youtube.com
12.3 Intermolecular Forces in Action Surface Tension & Viscosity YouTube Why Does Surface Tension Increase With Intermolecular Forces Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension the phenomenon that maintains the surface interface between liquid and. Why Does Surface Tension Increase With Intermolecular Forces.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Why Does Surface Tension Increase With Intermolecular Forces It allows you to place needles and insects on. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. Surface tension the phenomenon that maintains the surface interface between liquid and gas. Since these intermolecular forces vary. This property is caused by intermolecular forces, which keep the molecules. Surface. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Why Does Surface Tension Increase With Intermolecular Forces Since these intermolecular forces vary. It allows you to place needles and insects on. Surface tension the phenomenon that maintains the surface interface between liquid and gas. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. This property is caused by intermolecular forces,. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Does Surface Tension Increase With Intermolecular Forces Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. It allows you to place needles and insects on. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This property is caused by intermolecular forces, which keep the. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Section 5.6—Intermolecular Forces & Properties PowerPoint Why Does Surface Tension Increase With Intermolecular Forces Since these intermolecular forces vary. Surface tension the phenomenon that maintains the surface interface between liquid and gas. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This property is caused by intermolecular forces, which keep the molecules. Surface tension, the amount of energy required to increase the surface. Why Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Implication of Intermolecular Forces PowerPoint Presentation Why Does Surface Tension Increase With Intermolecular Forces Surface tension the phenomenon that maintains the surface interface between liquid and gas. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by. Why Does Surface Tension Increase With Intermolecular Forces.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru Why Does Surface Tension Increase With Intermolecular Forces Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. When your finger is pricked, a drop of blood forms and holds together due to surface tension—the. Why Does Surface Tension Increase With Intermolecular Forces.