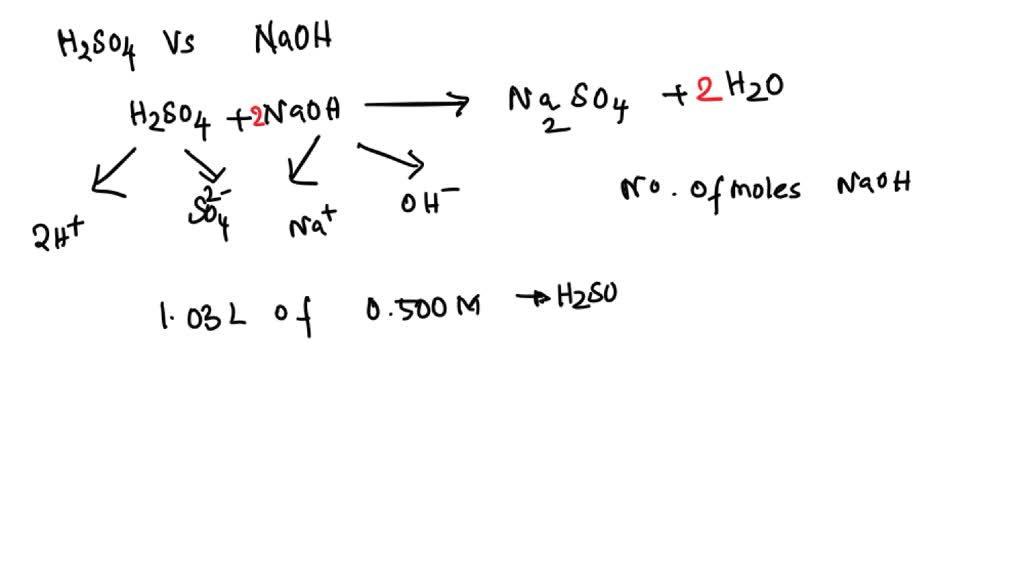
Titration Von H2So4 Mit Naoh . A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. If a third titration was required, average the two closest values. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Average the values for the total volumes of naoh added. My task is to now. I need to solve for the molarity of $\ce{h2so4}$. I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4).
from www.numerade.com
If a third titration was required, average the two closest values. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). Average the values for the total volumes of naoh added. I need to solve for the molarity of $\ce{h2so4}$. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). My task is to now. I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$.
SOLVED For the acidbase reaction (called titration) of sulfuric acid
Titration Von H2So4 Mit Naoh My task is to now. Average the values for the total volumes of naoh added. My task is to now. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). I need to solve for the molarity of $\ce{h2so4}$. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). If a third titration was required, average the two closest values.
From www.chegg.com
Solved Use the following titration curve for the first four Titration Von H2So4 Mit Naoh Average the values for the total volumes of naoh added. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. My task is to now. I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. I need to solve for the molarity of $\ce{h2so4}$. A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed.. Titration Von H2So4 Mit Naoh.
From www.studyhelp.de
Titration StudyHelp OnlineLernen Titration Von H2So4 Mit Naoh I need to solve for the molarity of $\ce{h2so4}$. I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. My task is to now. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. A titration. Titration Von H2So4 Mit Naoh.
From giotpdccx.blob.core.windows.net
H2So4 Titration With Naoh Calculation at Selene Larsen blog Titration Von H2So4 Mit Naoh $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. If a third titration was required, average the two closest values. I need to solve for the molarity of $\ce{h2so4}$. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). Average. Titration Von H2So4 Mit Naoh.
From www.numerade.com
SOLVED a titration was performed on a 25.0 mL sample of H2SO4 using 17 Titration Von H2So4 Mit Naoh I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. Average the values for the total volumes of naoh added. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. I need to solve for the molarity of $\ce{h2so4}$. My task is to now. If a third titration was required, average the two closest values. In this process, 2. Titration Von H2So4 Mit Naoh.
From chemistry291.blogspot.com
H2SO4 + NaOH Sulfuric acid(H2SO4) and Sodium hydroxide(NaOH)What is Titration Von H2So4 Mit Naoh This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). Average the values for the total volumes of naoh added. I need to solve for the molarity of $\ce{h2so4}$. My task is to now. In this process, 2 moles (the molecular weight of a substance expressed in. Titration Von H2So4 Mit Naoh.
From u-helmich.de
Chemie der Aminosäuren Titration Von H2So4 Mit Naoh A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. If a third titration was required, average the two closest values. My task is to now. I need to solve for the molarity of $\ce{h2so4}$. I am given $\ce{h2so4}$ in a reaction vessel of about. Titration Von H2So4 Mit Naoh.
From www.numerade.com
A student carried out a titration using H2SO4 and KOH. The balanced Titration Von H2So4 Mit Naoh In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na. Titration Von H2So4 Mit Naoh.
From www.vrogue.co
The Figure Shows The Ph Titration Curve Of 0 100 M Na vrogue.co Titration Von H2So4 Mit Naoh I need to solve for the molarity of $\ce{h2so4}$. My task is to now. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. In this process, 2 moles (the molecular weight of a substance expressed in. Titration Von H2So4 Mit Naoh.
From adelaideewawood.blogspot.com
H2so4 Naoh Balanced Equation AdelaideewaWood Titration Von H2So4 Mit Naoh If a third titration was required, average the two closest values. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Average the values for the total volumes of naoh added. A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. In this process, 2 moles (the molecular weight of a substance expressed in grams). Titration Von H2So4 Mit Naoh.
From www.chemikerboard.de
Aufgabe zur Titration Titration Von H2So4 Mit Naoh $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. My task is to now. I need to solve for the molarity of $\ce{h2so4}$. I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. In this process, 2 moles (the molecular weight of a. Titration Von H2So4 Mit Naoh.
From www.numerade.com
SOLVED Question 7 1 pt For the titration of sulfuric acid (H2SO4) with Titration Von H2So4 Mit Naoh My task is to now. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). Average the values for the total volumes of naoh added. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole. Titration Von H2So4 Mit Naoh.
From mungfali.com
The Titration Of 25 0 Ml Of An Unknown Concentration Of H2so4 Solution 699 Titration Von H2So4 Mit Naoh I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. I need to solve for the molarity of $\ce{h2so4}$. Average the values for the total volumes of naoh added. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). A titration using $\ce{naoh}$ (the same $\ce{naoh}$. Titration Von H2So4 Mit Naoh.
From www.scribd.com
Acid Base Titration Lab H2so4 + Naoh AP Chem 2 Titration Chemistry Titration Von H2So4 Mit Naoh $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). I need to solve for the molarity of $\ce{h2so4}$. My task is. Titration Von H2So4 Mit Naoh.
From www.numerade.com
SOLVED Consider the titration of sulfuric acid with sodium hydroxide Titration Von H2So4 Mit Naoh In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). If a third titration was required, average the two closest values. Average the values for the total volumes of naoh added. I am given $\ce{h2so4}$ in a reaction vessel of about. Titration Von H2So4 Mit Naoh.
From www.coursehero.com
[Solved] For the titration of sulfuric acid (H2SO4) with sodium hyd Titration Von H2So4 Mit Naoh Average the values for the total volumes of naoh added. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. If a third titration was required,. Titration Von H2So4 Mit Naoh.
From mavink.com
H2so4 Titration Curve Titration Von H2So4 Mit Naoh $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). If a third titration was required, average the two closest values. This results in the formation of two moles of water. Titration Von H2So4 Mit Naoh.
From www.youtube.com
How to balance NaOH + H2SO4→ Na2SO4+ H2O YouTube Titration Von H2So4 Mit Naoh Average the values for the total volumes of naoh added. I need to solve for the molarity of $\ce{h2so4}$. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. A titration. Titration Von H2So4 Mit Naoh.
From www.chegg.com
Solved 25.00mL of H2SO4 was titrated with 0.15M NaOH. Titration Von H2So4 Mit Naoh Average the values for the total volumes of naoh added. A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. I need to solve for the molarity of $\ce{h2so4}$. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). I am given. Titration Von H2So4 Mit Naoh.
From www.alamy.de
Titration mit HCl NaOH und Phenolphthalein hinzugefügt wird. Erste in Titration Von H2So4 Mit Naoh $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. My task is to now. If. Titration Von H2So4 Mit Naoh.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water Titration Von H2So4 Mit Naoh I need to solve for the molarity of $\ce{h2so4}$. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. If a third titration was required, average the two closest values. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). Average. Titration Von H2So4 Mit Naoh.
From researchersdays.science.lu
Wie funktioniert Titration? Titration Von H2So4 Mit Naoh Average the values for the total volumes of naoh added. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). I need to solve for the molarity of $\ce{h2so4}$. If a third titration was required, average the. Titration Von H2So4 Mit Naoh.
From www.chemielounge.de
Titration einer unbekannten H2SO4 Lösung Chemielounge Titration Von H2So4 Mit Naoh I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. If a third titration was required, average the two closest values. My task is to now. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of. Titration Von H2So4 Mit Naoh.
From www.numerade.com
SOLVED A0.256 M of H2SO4 was used to titrate 55.0 ml of NaOH 24.5 ml Titration Von H2So4 Mit Naoh My task is to now. If a third titration was required, average the two closest values. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. In this process, 2 moles (the molecular weight. Titration Von H2So4 Mit Naoh.
From giomzfpyz.blob.core.windows.net
Titration Of Naoh By H2So4 at Judith Phillips blog Titration Von H2So4 Mit Naoh My task is to now. Average the values for the total volumes of naoh added. I need to solve for the molarity of $\ce{h2so4}$. I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). In this. Titration Von H2So4 Mit Naoh.
From www.youtube.com
Neutralisation Reaction Sulfuric Acid and Sodium Hydroxide Balancing Titration Von H2So4 Mit Naoh My task is to now. Average the values for the total volumes of naoh added. I need to solve for the molarity of $\ce{h2so4}$. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). If a third titration was required, average the two closest values. A titration. Titration Von H2So4 Mit Naoh.
From www.numerade.com
SOLVED For the acidbase reaction (called titration) of sulfuric acid Titration Von H2So4 Mit Naoh I need to solve for the molarity of $\ce{h2so4}$. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). If a third titration was required, average the two closest values. This results in the formation of two moles of water (h. Titration Von H2So4 Mit Naoh.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration Von H2So4 Mit Naoh A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). Average the values for the total volumes of naoh added. I need to solve for the molarity of $\ce{h2so4}$. I am given. Titration Von H2So4 Mit Naoh.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water Titration Von H2So4 Mit Naoh In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). I need to solve for the molarity of $\ce{h2so4}$. A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. My task is to now. If a. Titration Von H2So4 Mit Naoh.
From www.abiweb.de
Mehrprotonige Säuren Chemie Titration Von H2So4 Mit Naoh My task is to now. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate. Titration Von H2So4 Mit Naoh.
From www.numerade.com
SOLVED Write a balanced equation for the corresponding titration Titration Von H2So4 Mit Naoh In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). If a third titration was required, average the two closest values. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na. Titration Von H2So4 Mit Naoh.
From www.vrogue.co
Standardization Of Naoh Acid Base Titration Objective vrogue.co Titration Von H2So4 Mit Naoh Average the values for the total volumes of naoh added. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in. Titration Von H2So4 Mit Naoh.
From www.chemielounge.de
Titration Schwefelsäure Berechnung der pHWerte der Äquivalenzpunkte Titration Von H2So4 Mit Naoh I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). If a third titration was. Titration Von H2So4 Mit Naoh.
From mavink.com
H2so4 Titration Curve Titration Von H2So4 Mit Naoh I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. I need to solve for the molarity of $\ce{h2so4}$. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). Average the values for the total volumes. Titration Von H2So4 Mit Naoh.
From giomzfpyz.blob.core.windows.net
Titration Of Naoh By H2So4 at Judith Phillips blog Titration Von H2So4 Mit Naoh A titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. Average the values for the total volumes of naoh added. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). If a third titration was required, average the two closest values. I. Titration Von H2So4 Mit Naoh.
From giomzfpyz.blob.core.windows.net
Titration Of Naoh By H2So4 at Judith Phillips blog Titration Von H2So4 Mit Naoh Average the values for the total volumes of naoh added. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. I am given $\ce{h2so4}$ in a reaction vessel of about $50~\mathrm{ml}$.. Titration Von H2So4 Mit Naoh.