An Engineer Wants To Estimate The Mass Of Gas . Determine the volume of the tank: 00 mol of the gas occupies a volume of 24. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. An engineer wants to estimate the mass of gas that is present in a tank. R is the ideal gas constant (approximately 0.0821 l·atm/mol·k), t is the temperature (in kelvin). She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. The engineer needs to measure the dimensions of the tank (length, width, and height) and calculate the. Using the number of moles. N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v is the. 1 calculate the number of moles of gas in the tank using the ideal gas law formula: An engineer wants to estimate the mass of gas that is present in a tank. An engineer wants to estimate the mass of gas that is present in a tank. The mass of the gas can be calculated using the number of moles and the molecular weight of the gas.
from www.chegg.com
00 mol of the gas occupies a volume of 24. An engineer wants to estimate the mass of gas that is present in a tank. R is the ideal gas constant (approximately 0.0821 l·atm/mol·k), t is the temperature (in kelvin). An engineer wants to estimate the mass of gas that is present in a tank. 1 calculate the number of moles of gas in the tank using the ideal gas law formula: An engineer wants to estimate the mass of gas that is present in a tank. The engineer needs to measure the dimensions of the tank (length, width, and height) and calculate the. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. Determine the volume of the tank: The mass of the gas can be calculated using the number of moles and the molecular weight of the gas.
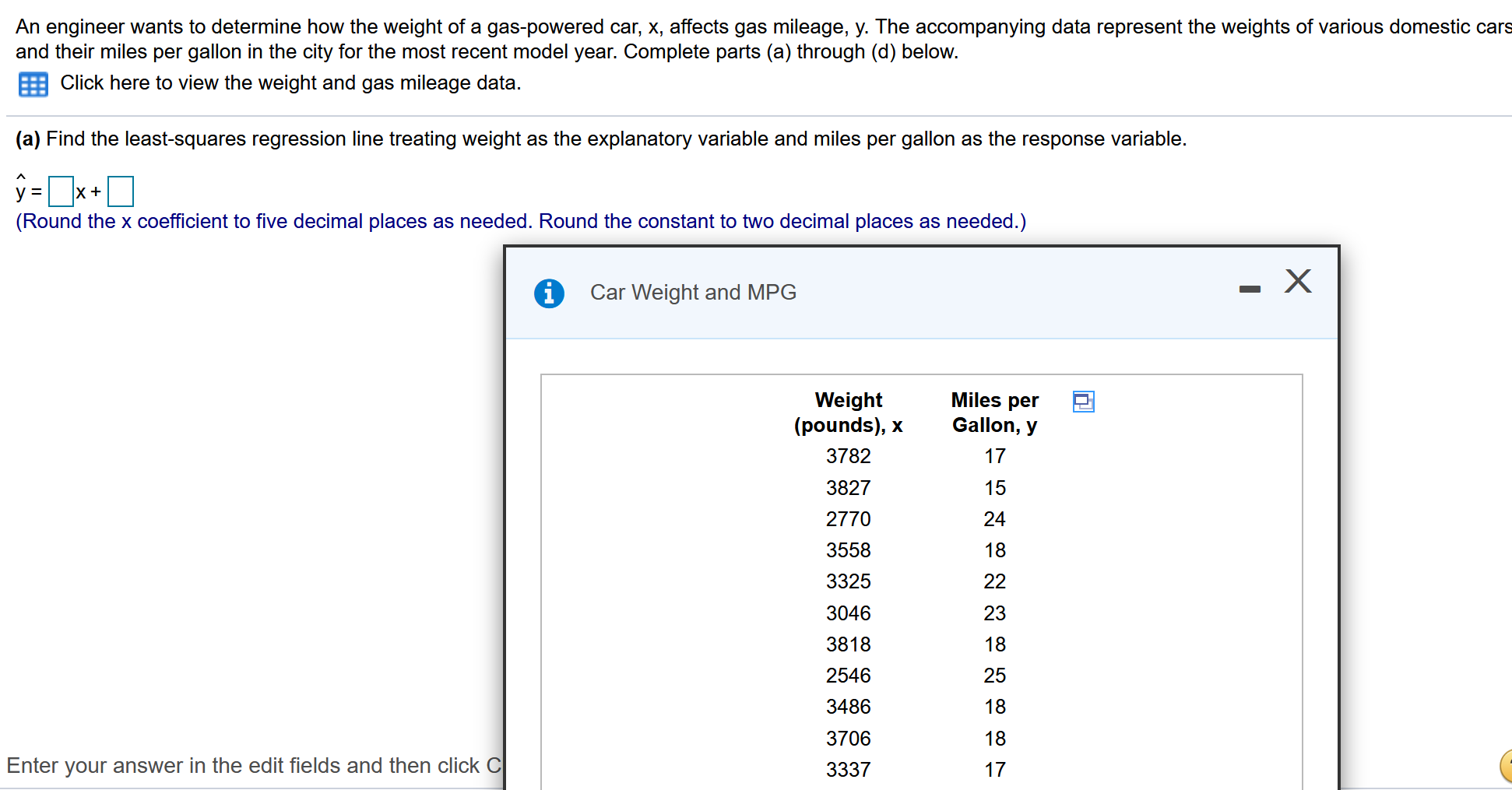
Solved An engineer wants to determine how the weight of a
An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. The engineer needs to measure the dimensions of the tank (length, width, and height) and calculate the. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. An engineer wants to estimate the mass of gas that is present in a tank. An engineer wants to estimate the mass of gas that is present in a tank. 1 calculate the number of moles of gas in the tank using the ideal gas law formula: 00 mol of the gas occupies a volume of 24. R is the ideal gas constant (approximately 0.0821 l·atm/mol·k), t is the temperature (in kelvin). The mass of the gas can be calculated using the number of moles and the molecular weight of the gas. N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v is the. Using the number of moles. Determine the volume of the tank: She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. An engineer wants to estimate the mass of gas that is present in a tank.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID6591194 An Engineer Wants To Estimate The Mass Of Gas An engineer wants to estimate the mass of gas that is present in a tank. The mass of the gas can be calculated using the number of moles and the molecular weight of the gas. R is the ideal gas constant (approximately 0.0821 l·atm/mol·k), t is the temperature (in kelvin). Determine the volume of the tank: An engineer wants to. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
Solved An engineer wants to determine how the weight of a An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. An engineer wants to estimate the mass of gas that is present in a tank. N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v is the. The mass of the gas can be calculated. An Engineer Wants To Estimate The Mass Of Gas.
From www.savemyexams.co.uk
Calculate Volumes of Gases (1.5.10) Edexcel IGCSE Chemistry Revision Notes 2019 Save My Exams An Engineer Wants To Estimate The Mass Of Gas The engineer needs to measure the dimensions of the tank (length, width, and height) and calculate the. An engineer wants to estimate the mass of gas that is present in a tank. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. The mass of the gas can be calculated using the number of. An Engineer Wants To Estimate The Mass Of Gas.
From www.youtube.com
Calculate volume of the gases liberated at STP if 1 L of 0.2 molar solution of ` YouTube An Engineer Wants To Estimate The Mass Of Gas An engineer wants to estimate the mass of gas that is present in a tank. N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v is the. Using the number of moles. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. 1 calculate the. An Engineer Wants To Estimate The Mass Of Gas.
From www.numerade.com
A chemical engineer wants to store a known amount of a useful gaseous byproduct from their plant An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. An engineer wants to estimate the mass of gas that is present in a tank. The mass of the gas can be calculated using the number of moles and the molecular weight of the gas. R is the ideal gas constant (approximately 0.0821 l·atm/mol·k),. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
Solved An environmental engineer wants to evaluate three An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. R is the ideal gas constant (approximately 0.0821 l·atm/mol·k), t is the temperature (in kelvin). The engineer needs to measure the dimensions of the tank (length, width, and height) and calculate the. 1 calculate the number of moles of gas in the tank using. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
Solved An engineer wants to determine how the weight of a An Engineer Wants To Estimate The Mass Of Gas An engineer wants to estimate the mass of gas that is present in a tank. Using the number of moles. Determine the volume of the tank: 00 mol of the gas occupies a volume of 24. N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v is the. The engineer needs. An Engineer Wants To Estimate The Mass Of Gas.
From www.youtube.com
PVM mRT Ideal Gas law 2 YouTube An Engineer Wants To Estimate The Mass Of Gas An engineer wants to estimate the mass of gas that is present in a tank. Using the number of moles. 1 calculate the number of moles of gas in the tank using the ideal gas law formula: The mass of the gas can be calculated using the number of moles and the molecular weight of the gas. The engineer needs. An Engineer Wants To Estimate The Mass Of Gas.
From www.youtube.com
Molar Volume of a Gas Lab Part 1 YouTube An Engineer Wants To Estimate The Mass Of Gas Determine the volume of the tank: An engineer wants to estimate the mass of gas that is present in a tank. 00 mol of the gas occupies a volume of 24. Using the number of moles. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. R is the ideal gas constant (approximately 0.0821. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
Solved An engineer wants to determine how the weight of a An Engineer Wants To Estimate The Mass Of Gas An engineer wants to estimate the mass of gas that is present in a tank. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. An engineer wants to estimate the mass of gas that is present in a tank. 00 mol of the gas occupies a volume of 24. An engineer wants to. An Engineer Wants To Estimate The Mass Of Gas.
From kunduz.com
[ANSWERED] An engineer wants to determine how the weight of a gas Kunduz An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. An engineer wants to estimate the mass of gas that is present in a tank. Using the number of moles. N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v is the. The engineer needs. An Engineer Wants To Estimate The Mass Of Gas.
From www.youtube.com
Using the Ideal Gas Law to find density or molar mass YouTube An Engineer Wants To Estimate The Mass Of Gas An engineer wants to estimate the mass of gas that is present in a tank. 1 calculate the number of moles of gas in the tank using the ideal gas law formula: An engineer wants to estimate the mass of gas that is present in a tank. Determine the volume of the tank: The engineer needs to measure the dimensions. An Engineer Wants To Estimate The Mass Of Gas.
From www.youtube.com
1.3 Calculating molar mass of a gas using PV=nRT YouTube An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. An engineer wants to estimate the mass of gas that is present in a tank. N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v is the. Determine the volume of the tank: An engineer. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
Solved An engineer wants to determine how the weight of a An Engineer Wants To Estimate The Mass Of Gas R is the ideal gas constant (approximately 0.0821 l·atm/mol·k), t is the temperature (in kelvin). N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v is the. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. The mass of the gas can be calculated. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
Solved A highway engineer wants to estimate the maximum An Engineer Wants To Estimate The Mass Of Gas Using the number of moles. The mass of the gas can be calculated using the number of moles and the molecular weight of the gas. An engineer wants to estimate the mass of gas that is present in a tank. An engineer wants to estimate the mass of gas that is present in a tank. She knows that 1.00 mol. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
Solved An engineer wants to determine how the weight of a An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. The engineer needs to measure the dimensions of the tank (length, width, and height) and calculate the. An engineer wants to estimate the mass of gas that is. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
Solved An engineer wants to determine how the weight of a An Engineer Wants To Estimate The Mass Of Gas 1 calculate the number of moles of gas in the tank using the ideal gas law formula: An engineer wants to estimate the mass of gas that is present in a tank. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. 00 mol of the gas occupies a volume of 24. Using the. An Engineer Wants To Estimate The Mass Of Gas.
From www.coursehero.com
[Solved] An engineer wants to determine how the weight of a gaspowered car,... Course Hero An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. Using the number of moles. 00 mol of the gas occupies a volume of 24. The engineer needs to measure the dimensions of the tank (length, width, and. An Engineer Wants To Estimate The Mass Of Gas.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial YouTube An Engineer Wants To Estimate The Mass Of Gas 00 mol of the gas occupies a volume of 24. The engineer needs to measure the dimensions of the tank (length, width, and height) and calculate the. The mass of the gas can be calculated using the number of moles and the molecular weight of the gas. An engineer wants to estimate the mass of gas that is present in. An Engineer Wants To Estimate The Mass Of Gas.
From www.youtube.com
11.6 The Combined Gas Law Pressure, Volume, & Temperature YouTube An Engineer Wants To Estimate The Mass Of Gas Using the number of moles. R is the ideal gas constant (approximately 0.0821 l·atm/mol·k), t is the temperature (in kelvin). She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v is the. An engineer wants. An Engineer Wants To Estimate The Mass Of Gas.
From www.youtube.com
4.17 Writing your own Basis for a mass balance calculation chemical engineering example YouTube An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. An engineer wants to estimate the mass of gas that is present in a tank. An engineer wants to estimate the mass of gas that is present in a tank. An engineer wants to estimate the mass of gas that is present in a. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
Solved An engineer wants to determine how the weight of a An Engineer Wants To Estimate The Mass Of Gas 00 mol of the gas occupies a volume of 24. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. An engineer wants to estimate the mass of gas that is present in a tank. 1 calculate the number of moles of gas in the tank using the ideal gas law formula: R is. An Engineer Wants To Estimate The Mass Of Gas.
From socratic.org
What is the mass, in kg, of 14.0 L of gasoline? (Density 0.680g/cm^3)? Socratic An Engineer Wants To Estimate The Mass Of Gas Using the number of moles. 1 calculate the number of moles of gas in the tank using the ideal gas law formula: The engineer needs to measure the dimensions of the tank (length, width, and height) and calculate the. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. An engineer wants to estimate. An Engineer Wants To Estimate The Mass Of Gas.
From en.ppt-online.org
The ideal gas equation online presentation An Engineer Wants To Estimate The Mass Of Gas An engineer wants to estimate the mass of gas that is present in a tank. The mass of the gas can be calculated using the number of moles and the molecular weight of the gas. An engineer wants to estimate the mass of gas that is present in a tank. R is the ideal gas constant (approximately 0.0821 l·atm/mol·k), t. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
In chemical engineering, spacetime is the time An Engineer Wants To Estimate The Mass Of Gas 00 mol of the gas occupies a volume of 24. An engineer wants to estimate the mass of gas that is present in a tank. R is the ideal gas constant (approximately 0.0821 l·atm/mol·k), t is the temperature (in kelvin). Determine the volume of the tank: Using the number of moles. The engineer needs to measure the dimensions of the. An Engineer Wants To Estimate The Mass Of Gas.
From kunduz.com
[ANSWERED] An engineer wants to determine how the weight of a gas Kunduz An Engineer Wants To Estimate The Mass Of Gas Determine the volume of the tank: Using the number of moles. R is the ideal gas constant (approximately 0.0821 l·atm/mol·k), t is the temperature (in kelvin). An engineer wants to estimate the mass of gas that is present in a tank. N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
Chemical Engineering Archive April 29, 2018 An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. 1 calculate the number of moles of gas in the tank using the ideal gas law formula: Using the number of moles. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. The mass of the gas can. An Engineer Wants To Estimate The Mass Of Gas.
From docslib.org
HOW to CALCULATE MASS PROPERTIES (An Engineer's Practical Guide) DocsLib An Engineer Wants To Estimate The Mass Of Gas The mass of the gas can be calculated using the number of moles and the molecular weight of the gas. Using the number of moles. 1 calculate the number of moles of gas in the tank using the ideal gas law formula: 00 mol of the gas occupies a volume of 24. An engineer wants to estimate the mass of. An Engineer Wants To Estimate The Mass Of Gas.
From www.numerade.com
SOLVED A chemical engineer must calculate the maximum safe operating temperature of a high An Engineer Wants To Estimate The Mass Of Gas 00 mol of the gas occupies a volume of 24. 1 calculate the number of moles of gas in the tank using the ideal gas law formula: The engineer needs to measure the dimensions of the tank (length, width, and height) and calculate the. The mass of the gas can be calculated using the number of moles and the molecular. An Engineer Wants To Estimate The Mass Of Gas.
From answerhappy.com
An engineer wants to determine how the weight of a gaspowered car, x, affects gas mileage, y An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v is the. An engineer wants to estimate the mass. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
Solved An engineer wants to determine how the weight of a An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v is the. The engineer needs to measure the dimensions of the tank (length, width, and height) and calculate the. An engineer wants to estimate the. An Engineer Wants To Estimate The Mass Of Gas.
From www.chegg.com
Solved An engineer wants to determine how the weight of a An Engineer Wants To Estimate The Mass Of Gas She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. Determine the volume of the tank: 1 calculate the number of moles of gas in the tank using the ideal gas law formula: An engineer wants to estimate the mass of gas that is present in a tank. An engineer wants to estimate the. An Engineer Wants To Estimate The Mass Of Gas.
From www.coursehero.com
[Solved] An engineer wants to determine how the weight of a gaspowered car,... Course Hero An Engineer Wants To Estimate The Mass Of Gas An engineer wants to estimate the mass of gas that is present in a tank. The mass of the gas can be calculated using the number of moles and the molecular weight of the gas. Determine the volume of the tank: She knows that 1.00 mol of the gas occupies a volume of 24.5 l at the. An engineer wants. An Engineer Wants To Estimate The Mass Of Gas.
From www.youtube.com
Ideal Gas Law Volume YouTube An Engineer Wants To Estimate The Mass Of Gas 00 mol of the gas occupies a volume of 24. Determine the volume of the tank: An engineer wants to estimate the mass of gas that is present in a tank. Using the number of moles. N = \frac {pv} {rt} n = rt p v , where p p is the pressure, v v is the. The engineer needs. An Engineer Wants To Estimate The Mass Of Gas.
From www.showme.com
Volume gas to Moles Science, Chemistry, Stoichiometry ShowMe An Engineer Wants To Estimate The Mass Of Gas 1 calculate the number of moles of gas in the tank using the ideal gas law formula: R is the ideal gas constant (approximately 0.0821 l·atm/mol·k), t is the temperature (in kelvin). An engineer wants to estimate the mass of gas that is present in a tank. Using the number of moles. An engineer wants to estimate the mass of. An Engineer Wants To Estimate The Mass Of Gas.