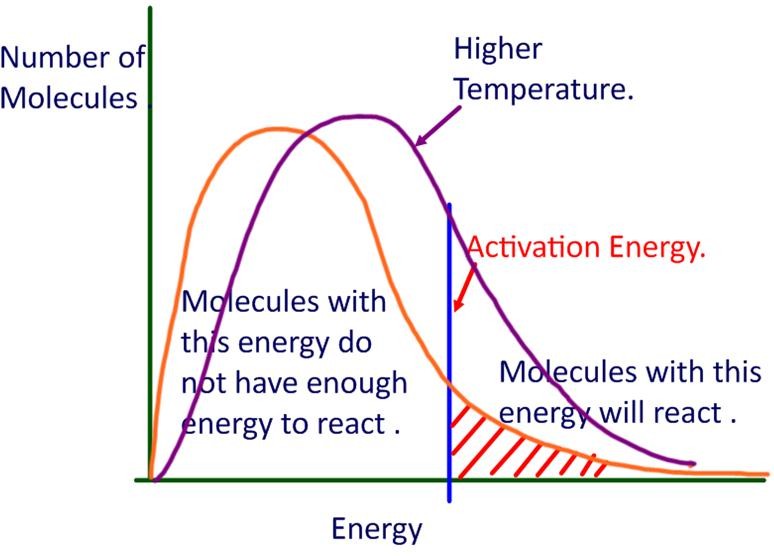
Threshold Energy In Collision Theory . For a chemical reaction to occur, an. Threshold energy is the minimum amount of energy needed for particles to react. In the graph (fig.8.3) 'e' corresponds. Only particles that have at least as. The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. They introduced a probability factor p to account for the effective collisions. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. They concluded that only those molecules that have the threshold energy (activation energy) and proper orientation during the collision will form products. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy. Define the concepts of activation energy and transition state. The rate of the reaction depends on the frequency of. For a particular reaction, the threshold energy might be as shown here:
from blogs.glowscotland.org.uk
For a chemical reaction to occur, an. Define the concepts of activation energy and transition state. Only particles that have at least as. Threshold energy is the minimum amount of energy needed for particles to react. In the graph (fig.8.3) 'e' corresponds. For a particular reaction, the threshold energy might be as shown here: They introduced a probability factor p to account for the effective collisions. The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur.
Collision Theory and Rate of Reaction Higher Chemistry Unit 1
Threshold Energy In Collision Theory Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Threshold energy is the minimum amount of energy needed for particles to react. The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). Only particles that have at least as. In the graph (fig.8.3) 'e' corresponds. The rate of the reaction depends on the frequency of. They concluded that only those molecules that have the threshold energy (activation energy) and proper orientation during the collision will form products. They introduced a probability factor p to account for the effective collisions. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. Define the concepts of activation energy and transition state. For a chemical reaction to occur, an. For a particular reaction, the threshold energy might be as shown here: Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy.
From courses.lumenlearning.com
Collision Theory Chemistry Threshold Energy In Collision Theory Define the concepts of activation energy and transition state. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. They introduced a probability factor p to account for the effective collisions. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The minimum amount of energy. Threshold Energy In Collision Theory.
From www.slideserve.com
PPT Chemical Reactions and Collision Theory PowerPoint Presentation, free download ID1833784 Threshold Energy In Collision Theory Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). Define the concepts of activation energy and transition state. Only particles that have at least as. For a chemical reaction to occur, an.. Threshold Energy In Collision Theory.
From www.studypool.com
SOLUTION Collision theory activation energy threshold energy arhenius equation catalyst Studypool Threshold Energy In Collision Theory The rate of the reaction depends on the frequency of. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. For a chemical reaction to occur, an. They introduced a probability factor p. Threshold Energy In Collision Theory.
From materiallibstaudt.z13.web.core.windows.net
Collision Theory Of Reactions Threshold Energy In Collision Theory For a chemical reaction to occur, an. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. They introduced a probability factor p to account for the effective collisions. For a particular reaction, the threshold energy might be as shown here: Define the concepts of activation energy and transition state. In the. Threshold Energy In Collision Theory.
From www.youtube.com
Collision theory threshold energy Activation energy IIT JEE NEET CBSE HBSE YouTube Threshold Energy In Collision Theory Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Define the concepts of activation energy and transition state. For a particular reaction, the threshold energy might be as shown here: Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Use the postulates of collision. Threshold Energy In Collision Theory.
From www.youtube.com
Collision Theory and Activation Energy YouTube Threshold Energy In Collision Theory Only particles that have at least as. For a chemical reaction to occur, an. They concluded that only those molecules that have the threshold energy (activation energy) and proper orientation during the collision will form products. Define the concepts of activation energy and transition state. For a particular reaction, the threshold energy might be as shown here: The minimum amount. Threshold Energy In Collision Theory.
From socratic.org
What is activation energy? What is threshold energy? What are the differences between them Threshold Energy In Collision Theory Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Threshold energy is the minimum amount of energy needed for particles to react. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. For a particular reaction, the threshold energy might be as shown here: In. Threshold Energy In Collision Theory.
From www.youtube.com
Chemical p7(activation energy,threshold energy,collision theory)by Kapil Dangi Sir Threshold Energy In Collision Theory Only particles that have at least as. They concluded that only those molecules that have the threshold energy (activation energy) and proper orientation during the collision will form products. The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy. Define the concepts of activation energy and. Threshold Energy In Collision Theory.
From www.youtube.com
Collision Theory and Activation Energy (ALevel IB Chemistry) YouTube Threshold Energy In Collision Theory Only particles that have at least as. The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy. For a particular reaction, the threshold energy might be as shown here: They introduced a probability factor p to account for the effective collisions. Collision theory states that for. Threshold Energy In Collision Theory.
From www.youtube.com
Chemical 14 Collision theory of chemical reaction, Activation energy and threshold Threshold Energy In Collision Theory Define the concepts of activation energy and transition state. Only particles that have at least as. The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). In the graph (fig.8.3) 'e' corresponds. For a chemical reaction to occur, an. Collision theory provides a qualitative explanation of chemical reactions and the rates. Threshold Energy In Collision Theory.
From www.coursehero.com
Collision Theory Chemistry Course Hero Threshold Energy In Collision Theory Only particles that have at least as. Threshold energy is the minimum amount of energy needed for particles to react. They concluded that only those molecules that have the threshold energy (activation energy) and proper orientation during the collision will form products. In the graph (fig.8.3) 'e' corresponds. For a chemical reaction to occur, an. Define the concepts of activation. Threshold Energy In Collision Theory.
From wiringfixsprang.z19.web.core.windows.net
Parts Of Collision Theory Threshold Energy In Collision Theory The rate of the reaction depends on the frequency of. Only particles that have at least as. They introduced a probability factor p to account for the effective collisions. For a chemical reaction to occur, an. The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy.. Threshold Energy In Collision Theory.
From slidetodoc.com
CHEM 3310 Chemical Collision Theory Transition State Threshold Energy In Collision Theory Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. They introduced a probability factor p to account for the effective collisions. Only particles that have at least as. In the graph (fig.8.3) 'e' corresponds. The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et).. Threshold Energy In Collision Theory.
From spmchemistry.blog.onlinetuition.com.my
Collision Theory SPM Chemistry Threshold Energy In Collision Theory Define the concepts of activation energy and transition state. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. For a particular reaction, the threshold energy might be as shown here: Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. They introduced a probability. Threshold Energy In Collision Theory.
From www.slideserve.com
PPT Chapter 18 Reaction Rates and Equilibrium PowerPoint Presentation ID3538891 Threshold Energy In Collision Theory Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Define the concepts of activation energy and transition state. Only particles that have at least as. For a chemical reaction to occur, an. The rate of the reaction depends on the frequency of. They introduced a probability factor p to account for. Threshold Energy In Collision Theory.
From www.revisechemistry.uk
Controlling reactions OCR Gateway C5 revisechemistry.uk Threshold Energy In Collision Theory In the graph (fig.8.3) 'e' corresponds. Define the concepts of activation energy and transition state. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. They concluded that only those molecules that have the threshold energy (activation energy) and proper orientation during the collision will form products. The minimum amount of. Threshold Energy In Collision Theory.
From www.youtube.com
How Collision Theory Relates to Equilibrium // HSC Chemistry YouTube Threshold Energy In Collision Theory Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy. Threshold energy is the minimum amount of energy needed for particles to react. They introduced a probability. Threshold Energy In Collision Theory.
From www.slideserve.com
PPT Collision Theory PowerPoint Presentation, free download ID3873529 Threshold Energy In Collision Theory Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Only particles that have at least as. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et).. Threshold Energy In Collision Theory.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME Threshold Energy In Collision Theory Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. They concluded that only those molecules that have the threshold energy (activation energy) and proper orientation during the collision will form products. For a chemical reaction. Threshold Energy In Collision Theory.
From blogs.glowscotland.org.uk
Collision Theory and Rate of Reaction Higher Chemistry Unit 1 Threshold Energy In Collision Theory Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy. Only particles that have at least as. Collision theory states that for a chemical reaction to occur,. Threshold Energy In Collision Theory.
From www.slideserve.com
PPT The Collision Theory and Activation Energy PowerPoint Presentation ID6544600 Threshold Energy In Collision Theory They concluded that only those molecules that have the threshold energy (activation energy) and proper orientation during the collision will form products. They introduced a probability factor p to account for the effective collisions. For a chemical reaction to occur, an. Define the concepts of activation energy and transition state. The minimum amount of energy which the colliding molecules must. Threshold Energy In Collision Theory.
From www.studypool.com
SOLUTION Collision theory activation energy threshold energy arhenius equation catalyst Studypool Threshold Energy In Collision Theory Threshold energy is the minimum amount of energy needed for particles to react. They concluded that only those molecules that have the threshold energy (activation energy) and proper orientation during the collision will form products. In the graph (fig.8.3) 'e' corresponds. The rate of the reaction depends on the frequency of. They introduced a probability factor p to account for. Threshold Energy In Collision Theory.
From www.pinterest.com
Simple Collision Theory Collision theory, Theories, Energy activities Threshold Energy In Collision Theory For a particular reaction, the threshold energy might be as shown here: They concluded that only those molecules that have the threshold energy (activation energy) and proper orientation during the collision will form products. Only particles that have at least as. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates.. Threshold Energy In Collision Theory.
From www.slideserve.com
PPT Chapter 18 Chemical Equilibrium PowerPoint Presentation, free download ID2089339 Threshold Energy In Collision Theory Define the concepts of activation energy and transition state. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. In the graph (fig.8.3) 'e' corresponds. For a particular reaction, the threshold energy might be as shown here: For a chemical reaction to occur, an. Only particles that have at least as.. Threshold Energy In Collision Theory.
From www.youtube.com
Collision Theory Arrhenius Equation & Activation Energy Chemical YouTube Threshold Energy In Collision Theory Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Define the concepts of activation energy and transition state. The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy. The rate of the reaction depends on the frequency. Threshold Energy In Collision Theory.
From www.slideserve.com
PPT Reaction Rate and Equilibrium PowerPoint Presentation, free download ID5660637 Threshold Energy In Collision Theory For a particular reaction, the threshold energy might be as shown here: In the graph (fig.8.3) 'e' corresponds. The rate of the reaction depends on the frequency of. The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). Threshold energy is the minimum amount of energy needed for particles to react.. Threshold Energy In Collision Theory.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Threshold Energy In Collision Theory Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. For a chemical reaction to occur, an. Threshold energy is the minimum amount of energy needed for particles to react. They introduced a probability factor p to account for the effective collisions. In the graph (fig.8.3) 'e' corresponds. Only particles that have at least. Threshold Energy In Collision Theory.
From www.studypool.com
SOLUTION Collision theory activation energy threshold energy arhenius equation catalyst Studypool Threshold Energy In Collision Theory Only particles that have at least as. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. Define the concepts of activation energy and transition state. The rate of the reaction depends on the frequency of. Collision theory states that for a chemical reaction to occur, the reacting particles must collide. Threshold Energy In Collision Theory.
From saylordotorg.github.io
The Collision Model of Chemical Threshold Energy In Collision Theory For a particular reaction, the threshold energy might be as shown here: In the graph (fig.8.3) 'e' corresponds. They concluded that only those molecules that have the threshold energy (activation energy) and proper orientation during the collision will form products. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Collision theory. Threshold Energy In Collision Theory.
From studylib.net
Collision Theory and Potential Energy Diagrams Threshold Energy In Collision Theory For a chemical reaction to occur, an. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Only particles that have at least as. The rate of the reaction depends on the frequency of. Threshold energy is the minimum amount of energy needed for particles to react. In the graph (fig.8.3) 'e'. Threshold Energy In Collision Theory.
From www.studypool.com
SOLUTION Collision theory activation energy threshold energy arhenius equation catalyst Studypool Threshold Energy In Collision Theory Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Define the concepts of activation energy and transition state. For a chemical reaction to occur, an. For a particular reaction, the threshold energy might be as shown here: Collision theory provides a qualitative explanation of chemical reactions and the rates at which. Threshold Energy In Collision Theory.
From www.youtube.com
Theory of rate of reaction Collision theory threshold energy Activation energy YouTube Threshold Energy In Collision Theory The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). For a particular reaction, the threshold energy might be as shown here: The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy. In the graph (fig.8.3) 'e'. Threshold Energy In Collision Theory.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Threshold Energy In Collision Theory Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction. Threshold Energy In Collision Theory.
From www.slideserve.com
PPT V. Reaction Rate (p. 562 565 & 568 570) PowerPoint Presentation ID1434280 Threshold Energy In Collision Theory For a chemical reaction to occur, an. The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). The rate of the reaction depends on the frequency of. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. In the graph (fig.8.3) 'e' corresponds. The minimum. Threshold Energy In Collision Theory.
From byjus.com
What happens when the energy of a reaction if more than threshold energy? Is such a situation Threshold Energy In Collision Theory The rate of the reaction depends on the frequency of. In the graph (fig.8.3) 'e' corresponds. They concluded that only those molecules that have the threshold energy (activation energy) and proper orientation during the collision will form products. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Only particles that have. Threshold Energy In Collision Theory.