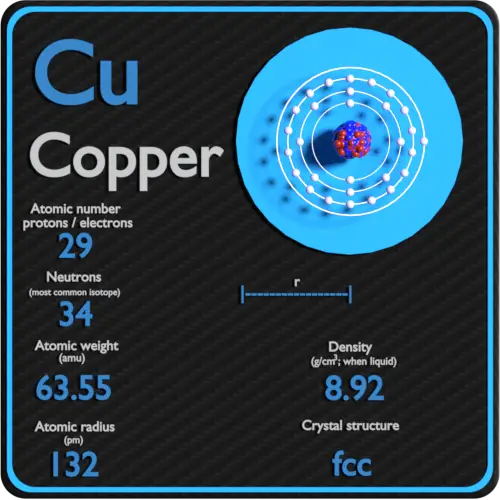
Copper (Atomic Mass 63.5) Occurs In Nature . Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Only two isotopes of copper occur naturally: Use this information to calculate the percent abundance of each copper. 63 cu (atomic mass = 62.9296 amu; Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. Use this information to calculate the percent abundance. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Its correctly recorded atomic mass (weighted average) is. Explain why the atomic mass of copper is not exactly.
from material-properties.org
Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. 63 cu (atomic mass = 62.9296 amu; Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Use this information to calculate the percent abundance of each copper. Only two isotopes of copper occur naturally: Explain why the atomic mass of copper is not exactly. Use this information to calculate the percent abundance. Its correctly recorded atomic mass (weighted average) is. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,.
Copper Periodic Table and Atomic Properties
Copper (Atomic Mass 63.5) Occurs In Nature Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. Use this information to calculate the percent abundance of each copper. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 63 cu (atomic mass = 62.9296 amu; Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Use this information to calculate the percent abundance. Only two isotopes of copper occur naturally: Its correctly recorded atomic mass (weighted average) is. Explain why the atomic mass of copper is not exactly.
From www.alamyimages.fr
Mendeleev periodic table copper Banque d'images vectorielles Alamy Copper (Atomic Mass 63.5) Occurs In Nature Use this information to calculate the percent abundance of each copper. 63 cu (atomic mass = 62.9296 amu; Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Explain why the atomic mass of copper is not exactly. Only two isotopes of copper occur naturally: Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass =. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.vrogue.co
The Average Atomic Mass Of Copper Is 63 546 Amu Natur vrogue.co Copper (Atomic Mass 63.5) Occurs In Nature Use this information to calculate the percent abundance of each copper. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Its correctly recorded atomic mass (weighted average) is. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Explain why the atomic mass of copper is not exactly. Only two isotopes of copper occur naturally: Copper has two isotopes, 63 cu. Copper (Atomic Mass 63.5) Occurs In Nature.
From sergbuster.weebly.com
Atomic mass of copper sergbuster Copper (Atomic Mass 63.5) Occurs In Nature Use this information to calculate the percent abundance of each copper. Only two isotopes of copper occur naturally: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.slideserve.com
PPT What is atomic mass? PowerPoint Presentation, free download ID Copper (Atomic Mass 63.5) Occurs In Nature Its correctly recorded atomic mass (weighted average) is. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. 63 cu (atomic mass = 62.9296 amu; Explain why the atomic mass of copper is not exactly. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.toppr.com
6. The density of copper is 9 x 103 kg/m and its atomic mass is 63.5 u Copper (Atomic Mass 63.5) Occurs In Nature Explain why the atomic mass of copper is not exactly. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Only two isotopes of copper occur naturally: 63 cu (atomic mass = 62.9296 amu; Use this information to calculate the percent abundance. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.alamy.com
Copper chemical element with first ionization energy, atomic mass and Copper (Atomic Mass 63.5) Occurs In Nature Use this information to calculate the percent abundance. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. Its correctly recorded atomic. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.chegg.com
Solved 1. Copper (A=63.5 g/mol) has an atomic radius of Copper (Atomic Mass 63.5) Occurs In Nature Explain why the atomic mass of copper is not exactly. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Use this information to calculate the percent abundance. Use this information to calculate the percent abundance of each copper. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Its correctly recorded atomic mass (weighted average) is. 63 cu (atomic mass =. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.numerade.com
SOLVEDNaturally occurring copper, Cu, is composed of two isotopes. The Copper (Atomic Mass 63.5) Occurs In Nature Explain why the atomic mass of copper is not exactly. Use this information to calculate the percent abundance. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Its correctly recorded atomic mass (weighted average) is. Use this information to calculate the percent abundance of each copper. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass =. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.slideserve.com
PPT BRONZE PowerPoint Presentation, free download ID3701702 Copper (Atomic Mass 63.5) Occurs In Nature Its correctly recorded atomic mass (weighted average) is. Only two isotopes of copper occur naturally: Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that. Copper (Atomic Mass 63.5) Occurs In Nature.
From utedzz.blogspot.com
Periodic Table Molar Mass Of Copper Periodic Table Timeline Copper (Atomic Mass 63.5) Occurs In Nature Use this information to calculate the percent abundance. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.shutterstock.com
2,277 Atomic Structure Copper Images, Stock Photos & Vectors Shutterstock Copper (Atomic Mass 63.5) Occurs In Nature Explain why the atomic mass of copper is not exactly. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 63 cu (atomic mass = 62.9296 amu; Use this information to calculate the percent abundance of each copper. Use this information to calculate the percent abundance. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Only two isotopes of copper occur. Copper (Atomic Mass 63.5) Occurs In Nature.
From material-properties.org
Copper Periodic Table and Atomic Properties Copper (Atomic Mass 63.5) Occurs In Nature Use this information to calculate the percent abundance. Use this information to calculate the percent abundance of each copper. Only two isotopes of copper occur naturally: Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Explain why the atomic mass of copper is not exactly. Its correctly recorded atomic mass (weighted average) is. 63 cu (atomic mass = 62.9296. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.numerade.com
SOLVED Copper has two isotopes, Cu63 and Cu65. The table below shows Copper (Atomic Mass 63.5) Occurs In Nature Use this information to calculate the percent abundance of each copper. 63 cu (atomic mass = 62.9296 amu; Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Use this information to calculate the percent abundance. Explain why the atomic mass of copper is not exactly. Its correctly recorded atomic mass (weighted average) is. Only two isotopes of copper occur naturally: Copper. Copper (Atomic Mass 63.5) Occurs In Nature.
From flowvella.com
Copper Screen 2 on FlowVella Presentation Software for Mac iPad and Copper (Atomic Mass 63.5) Occurs In Nature Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Explain why the atomic mass of copper is not exactly. Its correctly recorded atomic mass (weighted average) is. Use this information to calculate the percent abundance. Use this information to calculate the percent abundance of each copper. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass =. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID585423 Copper (Atomic Mass 63.5) Occurs In Nature 63 cu (atomic mass = 62.9296 amu; Only two isotopes of copper occur naturally: Explain why the atomic mass of copper is not exactly. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Use this information to calculate the percent abundance. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.dreamstime.com
Copper Chemical Element with First Ionization Energy, Atomic Mass and Copper (Atomic Mass 63.5) Occurs In Nature 63 cu (atomic mass = 62.9296 amu; Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. Sources, facts, uses, scarcity (sri),. Copper (Atomic Mass 63.5) Occurs In Nature.
From sanoberharald.blogspot.com
How To Work Out Relative Atomic Mass SanoberHarald Copper (Atomic Mass 63.5) Occurs In Nature Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. 63 cu (atomic mass = 62.9296 amu; Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single. Copper (Atomic Mass 63.5) Occurs In Nature.
From itechguide.pages.dev
Is Copper An Element itechguide Copper (Atomic Mass 63.5) Occurs In Nature 63 cu (atomic mass = 62.9296 amu; Only two isotopes of copper occur naturally: Use this information to calculate the percent abundance. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Its correctly recorded atomic mass (weighted average) is. Explain why the atomic mass of copper is not exactly. Copper has. Copper (Atomic Mass 63.5) Occurs In Nature.
From techiescientist.com
Is Copper an Element? Techiescientist Copper (Atomic Mass 63.5) Occurs In Nature Explain why the atomic mass of copper is not exactly. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Its correctly recorded atomic mass (weighted average) is. Use this information to calculate the percent abundance of each copper. 63 cu (atomic mass = 62.9296 amu; Use this information to calculate the percent abundance. Copper has two isotopes, 63 cu. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.dreamstime.com
Copper Atom, with Mass and Energy Levels. Stock Vector Illustration Copper (Atomic Mass 63.5) Occurs In Nature Its correctly recorded atomic mass (weighted average) is. Only two isotopes of copper occur naturally: Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper (Atomic Mass 63.5) Occurs In Nature Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Use this. Copper (Atomic Mass 63.5) Occurs In Nature.
From namacalne-szepty.blogspot.com
Electron Configuration Of Copper In Ground State namacalneszepty Copper (Atomic Mass 63.5) Occurs In Nature Its correctly recorded atomic mass (weighted average) is. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Use this information to calculate the percent abundance of each copper. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.alamy.com
Cu Copper Chemical Element Periodic Table. Single vector illustration Copper (Atomic Mass 63.5) Occurs In Nature Explain why the atomic mass of copper is not exactly. Only two isotopes of copper occur naturally: Use this information to calculate the percent abundance. Use this information to calculate the percent abundance of each copper. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are. Copper (Atomic Mass 63.5) Occurs In Nature.
From haipernews.com
How To Calculate Density Copper Haiper Copper (Atomic Mass 63.5) Occurs In Nature Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Explain why the atomic mass of copper is not exactly. Use this information to calculate the percent abundance. Its correctly recorded atomic mass (weighted average) is. Use this information to calculate the percent abundance of each copper. 63 cu (atomic mass = 62.9296 amu; Copper has two isotopes, 63 cu (69.15%, mass=62.9300. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.vrogue.co
The Average Atomic Mass Of Copper Is 63 546 Amu Natur vrogue.co Copper (Atomic Mass 63.5) Occurs In Nature Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Use this information to calculate the percent abundance of each copper. Use this information to calculate the percent abundance. Its correctly recorded atomic mass (weighted average) is. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass =. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.youtube.com
Calculating The Relative Atomic Mass of Copper GCSE Chemistry Copper (Atomic Mass 63.5) Occurs In Nature Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. Its correctly recorded atomic mass (weighted average) is. Only two isotopes of. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.coursehero.com
[Solved] What is the atomic mass of copper with isotopes copper63 with Copper (Atomic Mass 63.5) Occurs In Nature Only two isotopes of copper occur naturally: Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. Explain why the atomic mass. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.numerade.com
Copper has an average atomic mass of 63.55 amu and two naturally Copper (Atomic Mass 63.5) Occurs In Nature 63 cu (atomic mass = 62.9296 amu; Explain why the atomic mass of copper is not exactly. Its correctly recorded atomic mass (weighted average) is. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Only two isotopes of copper occur naturally: Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Use this information to calculate the percent abundance of each. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.slideserve.com
PPT Starter Quick Review PowerPoint Presentation, free download ID Copper (Atomic Mass 63.5) Occurs In Nature Use this information to calculate the percent abundance. Only two isotopes of copper occur naturally: Use this information to calculate the percent abundance of each copper. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.toppr.com
The average atomic mass of copper is 63.546 amu. Natural copper Copper (Atomic Mass 63.5) Occurs In Nature Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. Use this information to calculate the percent abundance of each copper. Sources,. Copper (Atomic Mass 63.5) Occurs In Nature.
From kunduz.com
[ANSWERED] The density of copper is 9 10 kg m and its atomic mass is 63 Copper (Atomic Mass 63.5) Occurs In Nature Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Explain why the atomic mass of copper is not exactly. Use this information to calculate the percent abundance of each copper. Its correctly recorded atomic mass (weighted average) is. Only two isotopes of copper occur naturally: Use this information to calculate the percent abundance. 63 cu (atomic mass = 62.9296 amu; Copper. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.pinterest.com
Copper Museumgrade sampleCopper Atomic Weight 63.546 Density 8.92 g Copper (Atomic Mass 63.5) Occurs In Nature 63 cu (atomic mass = 62.9296 amu; Its correctly recorded atomic mass (weighted average) is. Only two isotopes of copper occur naturally: Use this information to calculate the percent abundance of each copper. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu). Copper (Atomic Mass 63.5) Occurs In Nature.
From www.chegg.com
Solved Consider the following data for copper atomic mass Copper (Atomic Mass 63.5) Occurs In Nature Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. Explain why the atomic mass of copper is not exactly. Only two. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.sciencephoto.com
Copper, atomic structure Stock Image C045/6369 Science Photo Library Copper (Atomic Mass 63.5) Occurs In Nature Explain why the atomic mass of copper is not exactly. Use this information to calculate the percent abundance. Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Only two isotopes of copper occur naturally: Its correctly recorded atomic mass (weighted average) is. Use this information to calculate the percent abundance of each copper. 63 cu (atomic mass = 62.9296. Copper (Atomic Mass 63.5) Occurs In Nature.
From www.meritnation.com
Two isotopes of Copper, 63Cu has a mass of 62 930 u, and 65Cu has a Copper (Atomic Mass 63.5) Occurs In Nature Use this information to calculate the percent abundance of each copper. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Only two isotopes of copper occur naturally: Abundance 69.17 % and 65 (atomic mass = 64.9278 amu;. Its correctly recorded atomic mass (weighted average) is. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928. Copper (Atomic Mass 63.5) Occurs In Nature.