Lead Oxide And Sodium Hydroxide Reaction . Reaction of lead with bases. Lead dissolves slowly in cold alkalis to form plumbites. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. Pb (ii) reacts with hydroxide forming lead (ii) oxide. It describes the formation of lead (ii). Sodium oxide is a simple strongly basic oxide. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution.
from www.numerade.com
The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Reaction of lead with bases. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Pb (ii) reacts with hydroxide forming lead (ii) oxide. It describes the formation of lead (ii). \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. Sodium oxide is a simple strongly basic oxide. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:
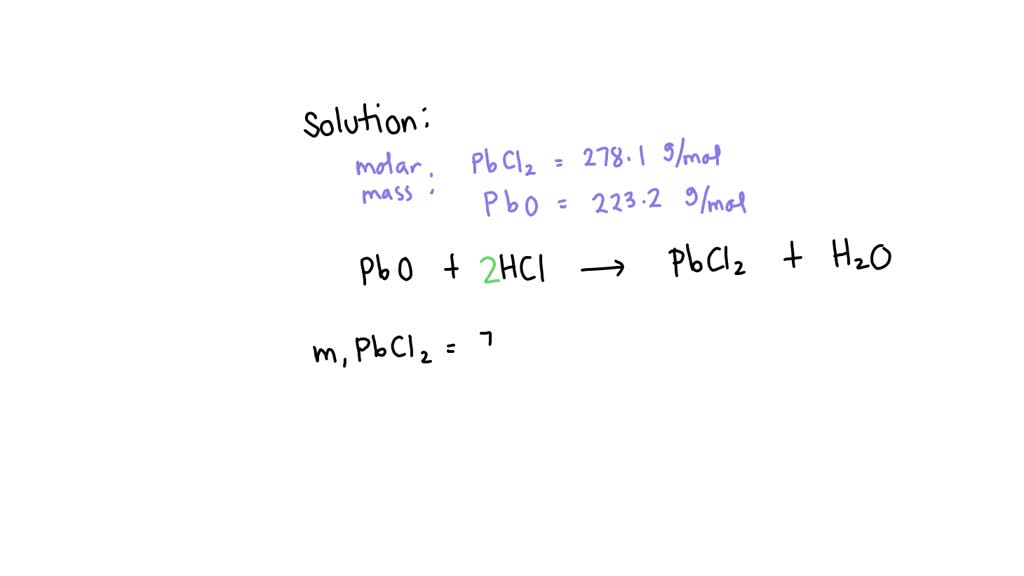
SOLVED Lead (II) oxide reacts with hydrochloric acid in a double
Lead Oxide And Sodium Hydroxide Reaction Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. It describes the formation of lead (ii). This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. Sodium oxide is a simple strongly basic oxide. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. Reaction of lead with bases. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Pb (ii) reacts with hydroxide forming lead (ii) oxide. Lead dissolves slowly in cold alkalis to form plumbites.
From www.toppr.com
Sodium Hyulum Lead oxide (? Checkuprogress! TITITI Choose the correct Lead Oxide And Sodium Hydroxide Reaction This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead dissolves slowly in cold alkalis to form plumbites. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather. Lead Oxide And Sodium Hydroxide Reaction.
From fyohzglop.blob.core.windows.net
Lead Sulphate And Sodium Hydroxide Reaction at Louise Johnson blog Lead Oxide And Sodium Hydroxide Reaction Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Lead dissolves slowly in cold alkalis to form plumbites. The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. Sodium oxide is a simple strongly basic oxide. This page discusses the precipitation of insoluble lead (ii). Lead Oxide And Sodium Hydroxide Reaction.
From www.youtube.com
Neutralisation Reaction Sulfuric Acid and Sodium Hydroxide Balancing Lead Oxide And Sodium Hydroxide Reaction This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. Reaction of lead with bases. Sodium oxide is a simple strongly basic oxide. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page looks at the formation of some insoluble lead (ii). Lead Oxide And Sodium Hydroxide Reaction.
From www.linstitute.net
CIE A Level Chemistry复习笔记2.2.2 Reactions of Group 2 Oxides, Hydroxides Lead Oxide And Sodium Hydroxide Reaction This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. Sodium oxide is a simple strongly basic oxide. Lead dissolves slowly in cold alkalis to form plumbites. This page looks at the formation of some insoluble lead (ii). Lead Oxide And Sodium Hydroxide Reaction.
From www.toppr.com
Sodium Hyulum Lead oxide (? Checkuprogress! TITITI Choose the correct Lead Oxide And Sodium Hydroxide Reaction It describes the formation of lead (ii). Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Reaction of lead with bases. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. This page looks at the formation of some insoluble lead (ii) compounds. Lead Oxide And Sodium Hydroxide Reaction.
From www.youtube.com
How to write the equation for PbO + H2O Lead (II) oxide + Water YouTube Lead Oxide And Sodium Hydroxide Reaction Lead dissolves slowly in cold alkalis to form plumbites. Sodium oxide is a simple strongly basic oxide. The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page discusses the precipitation of insoluble lead (ii). Lead Oxide And Sodium Hydroxide Reaction.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Lead Oxide And Sodium Hydroxide Reaction This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. Pb (ii) reacts with hydroxide forming lead (ii) oxide. Sodium oxide is a simple strongly basic oxide. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) +. Lead Oxide And Sodium Hydroxide Reaction.
From exohsvhug.blob.core.windows.net
Lead Oxide And Sodium Hydroxide Equation at Sebastian Malone blog Lead Oxide And Sodium Hydroxide Reaction Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Reaction of lead with. Lead Oxide And Sodium Hydroxide Reaction.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Lead Oxide And Sodium Hydroxide Reaction Lead dissolves slowly in cold alkalis to form plumbites. Reaction of lead with bases. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. This page looks at the formation of some insoluble lead (ii) compounds. Lead Oxide And Sodium Hydroxide Reaction.
From psu.pb.unizin.org
17.7 Electrolysis Chemistry 112 Chapters 1217 of OpenStax General Lead Oxide And Sodium Hydroxide Reaction Sodium oxide is a simple strongly basic oxide. Pb (ii) reacts with hydroxide forming lead (ii) oxide. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. Reaction of lead with bases. This page looks. Lead Oxide And Sodium Hydroxide Reaction.
From www.nagwa.com
Question Video Deducing the Balanced Chemical Equation for the Lead Oxide And Sodium Hydroxide Reaction \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. Lead dissolves slowly in cold alkalis to form plumbites. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: It describes the formation of lead (ii). This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using. Lead Oxide And Sodium Hydroxide Reaction.
From www.numerade.com
SOLVED Gaseous water and solid sodium oxide are produced by the Lead Oxide And Sodium Hydroxide Reaction Sodium oxide is a simple strongly basic oxide. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. Pb (ii) reacts with hydroxide forming lead (ii) oxide. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic. Lead Oxide And Sodium Hydroxide Reaction.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube Lead Oxide And Sodium Hydroxide Reaction Reaction of lead with bases. Sodium oxide is a simple strongly basic oxide. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Pb (ii) reacts with hydroxide forming lead (ii) oxide. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. \[\ce{pb^{2+}(aq). Lead Oxide And Sodium Hydroxide Reaction.
From trendzhit.com
Neutralisation Reaction Defination, Types, Equation, Applications Lead Oxide And Sodium Hydroxide Reaction This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Pb (ii) reacts with hydroxide forming lead (ii) oxide. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. It describes the formation of lead (ii). This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions. Lead Oxide And Sodium Hydroxide Reaction.
From exohsvhug.blob.core.windows.net
Lead Oxide And Sodium Hydroxide Equation at Sebastian Malone blog Lead Oxide And Sodium Hydroxide Reaction Sodium oxide is a simple strongly basic oxide. Reaction of lead with bases. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. This page looks at the. Lead Oxide And Sodium Hydroxide Reaction.
From byjus.com
The balanced net ionic equation for the reaction of aluminium sulphate Lead Oxide And Sodium Hydroxide Reaction Pb (ii) reacts with hydroxide forming lead (ii) oxide. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. The product of the reaction between pbo and naoh is lead (ii). Lead Oxide And Sodium Hydroxide Reaction.
From astonishingceiyrs.blogspot.com
Sodium Oxide Reacts With Water astonishingceiyrs Lead Oxide And Sodium Hydroxide Reaction This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. It describes the formation of lead (ii). Sodium oxide is a simple strongly basic oxide. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) +. Lead Oxide And Sodium Hydroxide Reaction.
From www.youtube.com
Write the balanced chemical equation for the reaction of sodium Lead Oxide And Sodium Hydroxide Reaction The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii). Lead Oxide And Sodium Hydroxide Reaction.
From teachernotes4u.com
Diagram shows the apparatus setup to study the reaction between sodium Lead Oxide And Sodium Hydroxide Reaction Reaction of lead with bases. Lead dissolves slowly in cold alkalis to form plumbites. Sodium oxide is a simple strongly basic oxide. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii). Lead Oxide And Sodium Hydroxide Reaction.
From www.slideserve.com
PPT The period 3 elements PowerPoint Presentation, free download ID Lead Oxide And Sodium Hydroxide Reaction Pb (ii) reacts with hydroxide forming lead (ii) oxide. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page looks at the formation of some insoluble lead (ii) compounds. Lead Oxide And Sodium Hydroxide Reaction.
From mariabsmitho.blob.core.windows.net
Lead Hydroxide Reaction Equation at mariabsmitho blog Lead Oxide And Sodium Hydroxide Reaction \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. Sodium oxide is a simple strongly basic oxide. Pb (ii) reacts with hydroxide forming lead (ii) oxide. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions. Lead Oxide And Sodium Hydroxide Reaction.
From exohsvhug.blob.core.windows.net
Lead Oxide And Sodium Hydroxide Equation at Sebastian Malone blog Lead Oxide And Sodium Hydroxide Reaction \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. Lead dissolves slowly in cold alkalis to form plumbites. The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. It describes the formation of lead (ii). This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. This page. Lead Oxide And Sodium Hydroxide Reaction.
From mammothmemory.net
Sodium reaction with hydrochloric acid is violent and quick Lead Oxide And Sodium Hydroxide Reaction \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. It describes the formation of lead (ii). This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Pb (ii) reacts with hydroxide forming lead (ii) oxide. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions. Lead Oxide And Sodium Hydroxide Reaction.
From www.youtube.com
Reaction of Sodium Hydroxide with Metals, Chemistry Lecture Sabaq.pk Lead Oxide And Sodium Hydroxide Reaction Lead dissolves slowly in cold alkalis to form plumbites. Pb (ii) reacts with hydroxide forming lead (ii) oxide. It describes the formation of lead (ii). The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. Sodium oxide is a simple strongly basic oxide. This page looks at the formation of some insoluble lead (ii) compounds. Lead Oxide And Sodium Hydroxide Reaction.
From www.chegg.com
Solved a Balance the reaction of sodium hydroxide and Lead Oxide And Sodium Hydroxide Reaction This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Pb (ii) reacts with hydroxide forming lead (ii) oxide. This page looks at the formation of some insoluble lead (ii) compounds. Lead Oxide And Sodium Hydroxide Reaction.
From www.youtube.com
OH Lewis Structure How to Draw the Lewis Dot Structure for the Lead Oxide And Sodium Hydroxide Reaction Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Lead dissolves slowly in cold alkalis to form plumbites. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. It describes the formation of lead (ii). The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. Sodium oxide is. Lead Oxide And Sodium Hydroxide Reaction.
From chemnotcheem.com
Redox reactions Oxidation and reduction O Level Chemistry Notes Lead Oxide And Sodium Hydroxide Reaction The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead dissolves slowly in cold alkalis to form. Lead Oxide And Sodium Hydroxide Reaction.
From www.alamy.com
Lead hydroxide precipitate formed by adding sodium hydroxide (NaOH) to Lead Oxide And Sodium Hydroxide Reaction Lead dissolves slowly in cold alkalis to form plumbites. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: It describes the formation of lead (ii). This page looks at the. Lead Oxide And Sodium Hydroxide Reaction.
From www.numerade.com
SOLVED Lead (II) oxide reacts with hydrochloric acid in a double Lead Oxide And Sodium Hydroxide Reaction Lead dissolves slowly in cold alkalis to form plumbites. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. Sodium oxide is a simple strongly basic oxide. The product of the reaction between. Lead Oxide And Sodium Hydroxide Reaction.
From www.numerade.com
SOLVED Gaseous water and solid sodium oxide are produced by the Lead Oxide And Sodium Hydroxide Reaction \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. It describes the formation of lead (ii). Sodium oxide is a simple strongly basic oxide. The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2. Lead dissolves slowly in cold alkalis. Lead Oxide And Sodium Hydroxide Reaction.
From www.slideserve.com
PPT Each reaction will usually be worth a total of 5 points Lead Oxide And Sodium Hydroxide Reaction This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. Sodium oxide is a. Lead Oxide And Sodium Hydroxide Reaction.
From openpress.usask.ca
11.2. Substitution Reactions SN2 Reactions Introduction to Organic Lead Oxide And Sodium Hydroxide Reaction This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. Pb (ii) reacts with. Lead Oxide And Sodium Hydroxide Reaction.
From www.youtube.com
Double displacement Pb(NO3)2 + NaOH Lead(II) nitrate + Sodium Lead Oxide And Sodium Hydroxide Reaction Lead dissolves slowly in cold alkalis to form plumbites. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Pb (ii) reacts with hydroxide forming lead (ii) oxide. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. The product of the reaction between pbo and naoh is lead (ii) hydroxide, pb (oh)2.. Lead Oxide And Sodium Hydroxide Reaction.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Lead Oxide And Sodium Hydroxide Reaction \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. Sodium oxide is a simple strongly basic oxide. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. It describes the formation of lead (ii). Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii). Lead Oxide And Sodium Hydroxide Reaction.
From www.chegg.com
Solved Reaction 1 The dissolving of solid sodium hydroxide Lead Oxide And Sodium Hydroxide Reaction Sodium oxide is a simple strongly basic oxide. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Reaction of lead with bases. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: The product of the reaction between pbo and. Lead Oxide And Sodium Hydroxide Reaction.