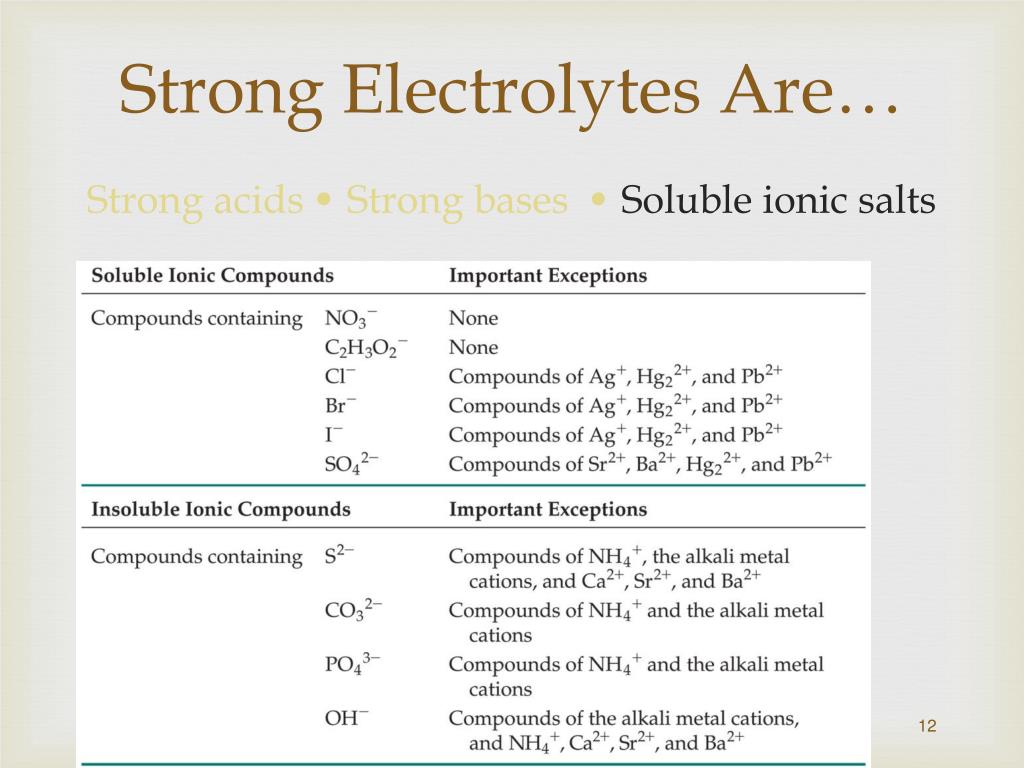
Table Salt Is Strong Electrolyte . However, many ionic compounds or salts of transition. Electrolytes can be classified as strong electrolytes and weak electrolytes. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Strong electrolytes are substances that completely. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. Yes, table salt contains electrolytes. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. The classic example is table salt or sodium chloride.
from www.slideserve.com
Strong electrolytes are substances that completely. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. However, many ionic compounds or salts of transition. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. The classic example is table salt or sodium chloride. Electrolytes can be classified as strong electrolytes and weak electrolytes. Yes, table salt contains electrolytes.
PPT Aqueous Reactions and Solution Stoichiometry PowerPoint
Table Salt Is Strong Electrolyte In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. Yes, table salt contains electrolytes. The classic example is table salt or sodium chloride. Strong electrolytes are substances that completely. Electrolytes can be classified as strong electrolytes and weak electrolytes. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. However, many ionic compounds or salts of transition. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte.
From www.nagwa.com
Question Video Identifying Which of Three Substances Is a Strong Table Salt Is Strong Electrolyte The classic example is table salt or sodium chloride. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. However, many ionic compounds or salts of transition. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID4763782 Table Salt Is Strong Electrolyte Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. Strong electrolytes are substances that completely. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. The classic example is. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I Table Salt Is Strong Electrolyte Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. Strong electrolytes are substances that completely. Yes, table salt contains electrolytes. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. The precise balance of minerals depends on where the salt was. Table Salt Is Strong Electrolyte.
From www.researchgate.net
List of Ions and Possible Aqueous Electrolytes in the SulfatePoor Table Salt Is Strong Electrolyte In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Strong electrolytes are substances that completely. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. The precise balance of minerals depends on where the. Table Salt Is Strong Electrolyte.
From www.vrogue.co
Electrolyte Nonelectrolyte Solutions Infographic Diag vrogue.co Table Salt Is Strong Electrolyte Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. The classic example is table salt or sodium chloride. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. Electrolytes can. Table Salt Is Strong Electrolyte.
From melissa-media.blogspot.com
Melissa Media Strong And Weak Electrolytes Table Salt Is Strong Electrolyte Strong electrolytes are substances that completely. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. The classic example is table salt or sodium chloride. However, many ionic compounds or salts of transition. In chemistry, a salt is an electrically neutral chemical compound consisting of. Table Salt Is Strong Electrolyte.
From slideserve.com
PPT II. Types of Electrolytes PowerPoint Presentation ID297972 Table Salt Is Strong Electrolyte However, many ionic compounds or salts of transition. Strong electrolytes are substances that completely. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. The classic example is table salt or sodium chloride. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary.. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free Table Salt Is Strong Electrolyte The classic example is table salt or sodium chloride. Electrolytes can be classified as strong electrolytes and weak electrolytes. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. Strong electrolytes are substances that completely. The precise balance of minerals depends on where the salt. Table Salt Is Strong Electrolyte.
From www.researchgate.net
Schematic illustration of three types of electrolytes. Download Table Salt Is Strong Electrolyte Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. The classic example is table salt or sodium chloride. Yes, table salt contains electrolytes. However, many ionic compounds or salts of transition. Standard issue. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Acid, Bases, and Salts PowerPoint Presentation, free download Table Salt Is Strong Electrolyte Strong electrolytes are substances that completely. Yes, table salt contains electrolytes. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Electrolytes and Nonelectrolytes PowerPoint Presentation, free Table Salt Is Strong Electrolyte Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. Strong electrolytes are substances that completely. Yes, table salt contains electrolytes. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. However, many ionic compounds. Table Salt Is Strong Electrolyte.
From ppt-online.org
Solutions. Equilibrium in solutions of strong and weak electrolytes Table Salt Is Strong Electrolyte Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. However, many ionic compounds or salts of transition. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Chemical reactions PowerPoint Presentation, free download ID Table Salt Is Strong Electrolyte Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. Strong electrolytes are substances that completely. Yes, table salt contains. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free Table Salt Is Strong Electrolyte Electrolytes can be classified as strong electrolytes and weak electrolytes. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. However, many ionic compounds or salts. Table Salt Is Strong Electrolyte.
From general.chemistrysteps.com
Strong and Weak Electrolytes Chemistry Steps Table Salt Is Strong Electrolyte Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Yes, table salt contains electrolytes. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Aqueous Reactions and Solution Stoichiometry PowerPoint Table Salt Is Strong Electrolyte The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. The classic example is table salt or sodium chloride. However, many ionic compounds or salts of transition. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Chemical reactions PowerPoint Presentation, free download ID Table Salt Is Strong Electrolyte The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. Electrolytes can be classified as strong electrolytes and weak electrolytes. However, many ionic compounds or salts of transition. Yes, table salt contains electrolytes. The classic example is table salt or sodium chloride. Many textbooks incorrectly state. Table Salt Is Strong Electrolyte.
From www.bouldersaltcompany.com
What Is the Best Salt For POTS? — Boulder Salt Company Table Salt Is Strong Electrolyte In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Yes, table salt contains electrolytes. Electrolytes can be classified as strong electrolytes and weak electrolytes. Strong electrolytes are substances that completely. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up. Table Salt Is Strong Electrolyte.
From courses.lumenlearning.com
Electrolytes Chemistry Table Salt Is Strong Electrolyte Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Water, Electrolytes, and Solutions PowerPoint Presentation, free Table Salt Is Strong Electrolyte However, many ionic compounds or salts of transition. Yes, table salt contains electrolytes. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. The classic example is table salt or sodium chloride. Electrolytes can be classified as strong electrolytes and weak electrolytes. In chemistry, a. Table Salt Is Strong Electrolyte.
From www.kentchemistry.com
Electrolytes Table Salt Is Strong Electrolyte The classic example is table salt or sodium chloride. Yes, table salt contains electrolytes. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. In chemistry, a salt is an electrically neutral chemical. Table Salt Is Strong Electrolyte.
From www.yourdictionary.com
Examples of Electrolytes Basic Explanation and Purpose YourDictionary Table Salt Is Strong Electrolyte In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Yes, table salt contains electrolytes. However, many ionic compounds or salts of transition. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. The precise. Table Salt Is Strong Electrolyte.
From scottscience.weebly.com
Unit 11 Solutions Table Salt Is Strong Electrolyte Electrolytes can be classified as strong electrolytes and weak electrolytes. However, many ionic compounds or salts of transition. Strong electrolytes are substances that completely. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary.. Table Salt Is Strong Electrolyte.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Table Salt Is Strong Electrolyte Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. The classic example is table salt or sodium chloride. Electrolytes can be classified as strong electrolytes and weak electrolytes. Yes, table salt contains electrolytes. The precise balance of minerals depends on where the salt was. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Electrolytes & Nonelectrolytes PowerPoint Presentation, free Table Salt Is Strong Electrolyte Yes, table salt contains electrolytes. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. The classic example is table salt or sodium chloride. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. However, many ionic compounds or salts of transition. Electrolytes. Table Salt Is Strong Electrolyte.
From studiousguy.com
9 Electrolyte Examples in Daily Life StudiousGuy Table Salt Is Strong Electrolyte Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. However, many ionic compounds or salts of transition. Electrolytes can be classified as strong electrolytes. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Aqueoussolution Reactions PowerPoint Presentation, free download Table Salt Is Strong Electrolyte Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. However, many ionic compounds or salts of transition. Electrolytes can be classified as strong electrolytes and weak electrolytes. In chemistry, a salt is. Table Salt Is Strong Electrolyte.
From thecontentauthority.com
Salt vs Electrolyte Decoding Common Word MixUps Table Salt Is Strong Electrolyte Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. However, many ionic compounds or salts of transition. Strong electrolytes are substances that completely. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. Sodium and chloride are electrolytes, responsible for maintaining the. Table Salt Is Strong Electrolyte.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Table Salt Is Strong Electrolyte The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. Yes, table salt contains electrolytes. Sodium and chloride are electrolytes, responsible. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Chapter 4 Reactions in Aqueous Solutions PowerPoint Presentation Table Salt Is Strong Electrolyte Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. Yes, table salt contains electrolytes. The classic example is table salt or sodium chloride. In chemistry, a salt is an electrically neutral chemical. Table Salt Is Strong Electrolyte.
From saltsanity.com
How Much Sodium is in Table Salt? Tips for Low Sodium Living Table Salt Is Strong Electrolyte However, many ionic compounds or salts of transition. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great. Table Salt Is Strong Electrolyte.
From www.slideserve.com
PPT Post Lab Electrolytes PowerPoint Presentation, free download Table Salt Is Strong Electrolyte Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. Electrolytes can be classified as strong electrolytes and weak electrolytes. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Yes, table salt contains electrolytes. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte.. Table Salt Is Strong Electrolyte.
From www.aakash.ac.in
Electrolytes and Nonelectrolytes Types, Differences & Examples AESL Table Salt Is Strong Electrolyte Strong electrolytes are substances that completely. The classic example is table salt or sodium chloride. Electrolytes can be classified as strong electrolytes and weak electrolytes. However, many ionic compounds or salts of transition. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. Standard issue. Table Salt Is Strong Electrolyte.
From www.majordifferences.com
Strong Electrolytes vs Weak Electrolytes Major Differences Table Salt Is Strong Electrolyte Strong electrolytes are substances that completely. The precise balance of minerals depends on where the salt was harvested, but generally they include a combination of silicon and phosphorus (both necessary. Yes, table salt contains electrolytes. However, many ionic compounds or salts of transition. Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. The classic example is. Table Salt Is Strong Electrolyte.
From pediaa.com
Difference Between Strong and Weak Electrolytes Definition Table Salt Is Strong Electrolyte Many textbooks incorrectly state that all salts or ionic compounds are strong electrolytes. Yes, table salt contains electrolytes. Strong electrolytes are substances that completely. Sodium and chloride are electrolytes, responsible for maintaining the body’s overall electrolyte. However, many ionic compounds or salts of transition. The classic example is table salt or sodium chloride. The precise balance of minerals depends on. Table Salt Is Strong Electrolyte.