What Is A Mixture Of Metals Called . For example, brass is an. Mixtures can be solids, liquids, gases, or a combination of states of. Alloys are mixtures of metals or a mixture of a metal and another element. You might find the idea of an alloy as a mixture of metals quite confusing. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Most alloys form by melting the elements together. The traditional way of making alloys was to heat. A mixture consists of two or more chemically distinct components that do not react with each other. The structure of metals explains their high melting and boiling points and their conductivity. Alloys can be formed by substituting one. An alloy may be a solid solution of metal elements (a. The properties of a metal can be modified by mixing it with another substance to form an alloy. An alloy is a substance made by combining together two or more elements where the primary element is a metal. How can you mix together two lumps of solid metal?
from general.chemistrysteps.com
Most alloys form by melting the elements together. Mixtures can be solids, liquids, gases, or a combination of states of. A mixture consists of two or more chemically distinct components that do not react with each other. How can you mix together two lumps of solid metal? An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Alloys can be formed by substituting one. For example, brass is an. An alloy may be a solid solution of metal elements (a. Alloys are mixtures of metals or a mixture of a metal and another element. An alloy is a substance made by combining together two or more elements where the primary element is a metal.
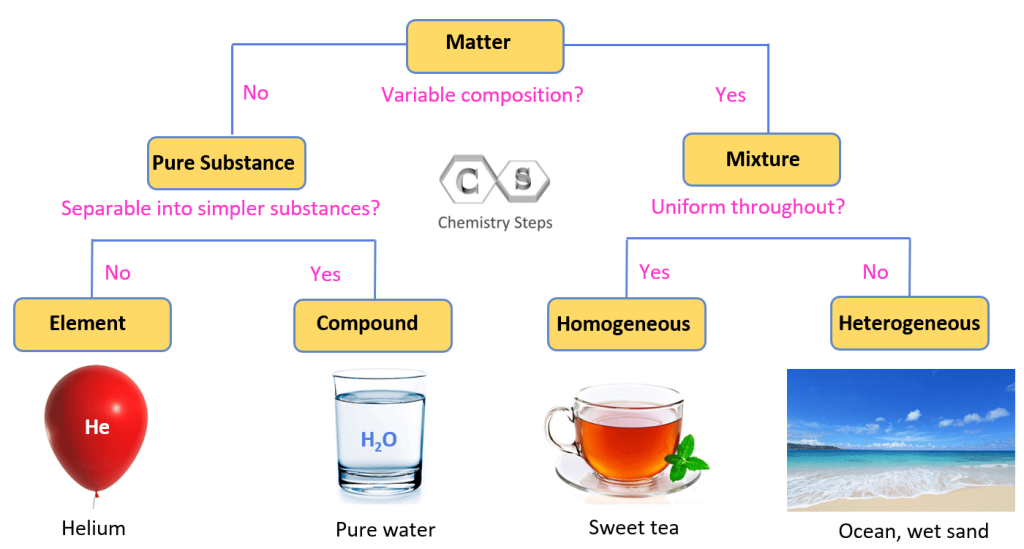
Pure Substances, Mixtures, Elements, and Compounds Chemistry Steps
What Is A Mixture Of Metals Called The properties of a metal can be modified by mixing it with another substance to form an alloy. A mixture consists of two or more chemically distinct components that do not react with each other. The traditional way of making alloys was to heat. Most alloys form by melting the elements together. Mixtures can be solids, liquids, gases, or a combination of states of. An alloy is a substance made by combining together two or more elements where the primary element is a metal. For example, brass is an. The structure of metals explains their high melting and boiling points and their conductivity. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. You might find the idea of an alloy as a mixture of metals quite confusing. An alloy may be a solid solution of metal elements (a. Alloys are mixtures of metals or a mixture of a metal and another element. How can you mix together two lumps of solid metal? Alloys can be formed by substituting one. The properties of a metal can be modified by mixing it with another substance to form an alloy.
From www.slideserve.com
PPT I S M A T T E R A R O U N D U S P U R E ? ? ? ? ? PowerPoint What Is A Mixture Of Metals Called An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Alloys can be formed by substituting one. How can you mix together two lumps of solid metal? A mixture consists of two or more chemically distinct components that do not react with each other. You might find the idea of an. What Is A Mixture Of Metals Called.
From www.slideshare.net
Elements, Compounds, Mixtures What Is A Mixture Of Metals Called The properties of a metal can be modified by mixing it with another substance to form an alloy. Most alloys form by melting the elements together. An alloy is a substance made by combining together two or more elements where the primary element is a metal. For example, brass is an. A mixture consists of two or more chemically distinct. What Is A Mixture Of Metals Called.
From www.slideserve.com
PPT Elements, Compounds and Mixtures PowerPoint Presentation, free What Is A Mixture Of Metals Called A mixture consists of two or more chemically distinct components that do not react with each other. The traditional way of making alloys was to heat. Alloys can be formed by substituting one. You might find the idea of an alloy as a mixture of metals quite confusing. An alloy may be a solid solution of metal elements (a. Most. What Is A Mixture Of Metals Called.
From www.slideserve.com
PPT Mixtures and Solutions PowerPoint Presentation, free download What Is A Mixture Of Metals Called Most alloys form by melting the elements together. An alloy is a substance made by combining together two or more elements where the primary element is a metal. The properties of a metal can be modified by mixing it with another substance to form an alloy. For example, brass is an. Alloys are mixtures of metals or a mixture of. What Is A Mixture Of Metals Called.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures What Is A Mixture Of Metals Called Alloys are mixtures of metals or a mixture of a metal and another element. An alloy is a substance made by combining together two or more elements where the primary element is a metal. You might find the idea of an alloy as a mixture of metals quite confusing. A mixture consists of two or more chemically distinct components that. What Is A Mixture Of Metals Called.
From slideplayer.com
MIXTURES. ppt download What Is A Mixture Of Metals Called An alloy is a substance made by combining together two or more elements where the primary element is a metal. The properties of a metal can be modified by mixing it with another substance to form an alloy. For example, brass is an. The traditional way of making alloys was to heat. You might find the idea of an alloy. What Is A Mixture Of Metals Called.
From slideplayer.com
Chem 1 Chapter 8 Ionic Bonding ppt download What Is A Mixture Of Metals Called A mixture consists of two or more chemically distinct components that do not react with each other. The structure of metals explains their high melting and boiling points and their conductivity. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. For example, brass is an. Alloys are mixtures of metals. What Is A Mixture Of Metals Called.
From www.slideserve.com
PPT Entrance Question PowerPoint Presentation, free download ID2799310 What Is A Mixture Of Metals Called Alloys can be formed by substituting one. An alloy is a substance made by combining together two or more elements where the primary element is a metal. Most alloys form by melting the elements together. Mixtures can be solids, liquids, gases, or a combination of states of. For example, brass is an. The properties of a metal can be modified. What Is A Mixture Of Metals Called.
From slidetodoc.com
3 3 METALS Metals on the Periodic Table What Is A Mixture Of Metals Called For example, brass is an. How can you mix together two lumps of solid metal? You might find the idea of an alloy as a mixture of metals quite confusing. The traditional way of making alloys was to heat. Most alloys form by melting the elements together. Alloys can be formed by substituting one. Alloys are mixtures of metals or. What Is A Mixture Of Metals Called.
From whatisdiffer.com
What Is The Difference Between Alloys And Pure Metals? What Is A Mixture Of Metals Called For example, brass is an. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. How can you mix together two lumps of solid metal? An alloy may be a solid solution of metal elements (a. Mixtures can be solids, liquids, gases, or a combination of states of. The properties of. What Is A Mixture Of Metals Called.
From studiousguy.com
Alloys Properties & Uses StudiousGuy What Is A Mixture Of Metals Called You might find the idea of an alloy as a mixture of metals quite confusing. An alloy is a substance made by combining together two or more elements where the primary element is a metal. The properties of a metal can be modified by mixing it with another substance to form an alloy. An alloy may be a solid solution. What Is A Mixture Of Metals Called.
From smartclass4kids.com
What is Mixture, Homogeneous Mixture, Heterogeneous Mixture with Examples What Is A Mixture Of Metals Called The structure of metals explains their high melting and boiling points and their conductivity. Mixtures can be solids, liquids, gases, or a combination of states of. The properties of a metal can be modified by mixing it with another substance to form an alloy. Alloys are mixtures of metals or a mixture of a metal and another element. An alloy. What Is A Mixture Of Metals Called.
From www.slideserve.com
PPT METAL ALLOYS (Homogeneous Mixtures!) PowerPoint Presentation What Is A Mixture Of Metals Called The traditional way of making alloys was to heat. For example, brass is an. Alloys can be formed by substituting one. The properties of a metal can be modified by mixing it with another substance to form an alloy. How can you mix together two lumps of solid metal? Most alloys form by melting the elements together. Mixtures can be. What Is A Mixture Of Metals Called.
From www.thoughtco.com
Properties, Composition, and Production of Metal Alloys What Is A Mixture Of Metals Called The properties of a metal can be modified by mixing it with another substance to form an alloy. Alloys are mixtures of metals or a mixture of a metal and another element. The structure of metals explains their high melting and boiling points and their conductivity. An alloy is a substance made by combining together two or more elements where. What Is A Mixture Of Metals Called.
From www.youtube.com
21.1 Alloys Metallic Mixtures YouTube What Is A Mixture Of Metals Called Most alloys form by melting the elements together. Alloys are mixtures of metals or a mixture of a metal and another element. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. You might find the idea of an alloy as a mixture of metals quite confusing. For example, brass is. What Is A Mixture Of Metals Called.
From www.youtube.com
MIXING METALS MOLTEN BRASS ALUMINIUM & LEAD TOGETHER AT THE SAME TIME What Is A Mixture Of Metals Called How can you mix together two lumps of solid metal? Alloys are mixtures of metals or a mixture of a metal and another element. You might find the idea of an alloy as a mixture of metals quite confusing. A mixture consists of two or more chemically distinct components that do not react with each other. The properties of a. What Is A Mixture Of Metals Called.
From general.chemistrysteps.com
Pure Substances, Mixtures, Elements, and Compounds Chemistry Steps What Is A Mixture Of Metals Called Most alloys form by melting the elements together. The traditional way of making alloys was to heat. For example, brass is an. The structure of metals explains their high melting and boiling points and their conductivity. Mixtures can be solids, liquids, gases, or a combination of states of. You might find the idea of an alloy as a mixture of. What Is A Mixture Of Metals Called.
From atscience.weebly.com
Elements, Compounds and Mixtures AT SCIENCE What Is A Mixture Of Metals Called Most alloys form by melting the elements together. The structure of metals explains their high melting and boiling points and their conductivity. An alloy is a substance made by combining together two or more elements where the primary element is a metal. For example, brass is an. The properties of a metal can be modified by mixing it with another. What Is A Mixture Of Metals Called.
From www.slideserve.com
PPT Pure substances and mixtures PowerPoint Presentation ID2262547 What Is A Mixture Of Metals Called The properties of a metal can be modified by mixing it with another substance to form an alloy. The structure of metals explains their high melting and boiling points and their conductivity. How can you mix together two lumps of solid metal? A mixture consists of two or more chemically distinct components that do not react with each other. Most. What Is A Mixture Of Metals Called.
From www.slideshare.net
Elements, Compounds, Mixtures What Is A Mixture Of Metals Called The structure of metals explains their high melting and boiling points and their conductivity. For example, brass is an. A mixture consists of two or more chemically distinct components that do not react with each other. Alloys are mixtures of metals or a mixture of a metal and another element. An alloy may be a solid solution of metal elements. What Is A Mixture Of Metals Called.
From www.youtube.com
The MetalsMixtures and Solutions YouTube What Is A Mixture Of Metals Called The structure of metals explains their high melting and boiling points and their conductivity. Alloys are mixtures of metals or a mixture of a metal and another element. The properties of a metal can be modified by mixing it with another substance to form an alloy. An alloy is a mixture of metals that has bulk metallic properties different from. What Is A Mixture Of Metals Called.
From matsciwit.blogspot.com
Materials Witness The Elemental Composition of Metal Alloys What Is A Mixture Of Metals Called An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. The properties of a metal can be modified by mixing it with another substance to form an alloy. Mixtures can be solids, liquids, gases, or a combination of states of. For example, brass is an. An alloy may be a solid. What Is A Mixture Of Metals Called.
From science4geeks.blogspot.com
Science4Geeks Element, Compound and Mixture What Is A Mixture Of Metals Called Most alloys form by melting the elements together. The structure of metals explains their high melting and boiling points and their conductivity. The traditional way of making alloys was to heat. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. How can you mix together two lumps of solid metal?. What Is A Mixture Of Metals Called.
From belengrolevy.blogspot.com
What Are Metals That Have Been Mixed Together Called What Is A Mixture Of Metals Called Alloys are mixtures of metals or a mixture of a metal and another element. You might find the idea of an alloy as a mixture of metals quite confusing. The properties of a metal can be modified by mixing it with another substance to form an alloy. Alloys can be formed by substituting one. The traditional way of making alloys. What Is A Mixture Of Metals Called.
From www.sliderbase.com
Elements compounds and mixtures Presentation Chemistry What Is A Mixture Of Metals Called An alloy may be a solid solution of metal elements (a. The properties of a metal can be modified by mixing it with another substance to form an alloy. Alloys can be formed by substituting one. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. A mixture consists of two. What Is A Mixture Of Metals Called.
From www.slideserve.com
PPT 3/27 Notes on Mixtures PowerPoint Presentation, free download What Is A Mixture Of Metals Called Mixtures can be solids, liquids, gases, or a combination of states of. Most alloys form by melting the elements together. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. The traditional way of making alloys was to heat. The properties of a metal can be modified by mixing it with. What Is A Mixture Of Metals Called.
From www.slideshare.net
Metals What Is A Mixture Of Metals Called The properties of a metal can be modified by mixing it with another substance to form an alloy. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. An alloy may be a solid solution of metal elements (a. For example, brass is an. You might find the idea of an. What Is A Mixture Of Metals Called.
From slideplayer.com
Metals, Nonmetals and Metalloids ppt download What Is A Mixture Of Metals Called Alloys are mixtures of metals or a mixture of a metal and another element. An alloy may be a solid solution of metal elements (a. The traditional way of making alloys was to heat. The structure of metals explains their high melting and boiling points and their conductivity. How can you mix together two lumps of solid metal? The properties. What Is A Mixture Of Metals Called.
From engineeringlearn.com
16 Types of Metals and Their Uses [with Pictures] Engineering Learn What Is A Mixture Of Metals Called A mixture consists of two or more chemically distinct components that do not react with each other. Mixtures can be solids, liquids, gases, or a combination of states of. Most alloys form by melting the elements together. An alloy may be a solid solution of metal elements (a. For example, brass is an. An alloy is a mixture of metals. What Is A Mixture Of Metals Called.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 2.26C Know that an Alloy is a Mixture of a Metal What Is A Mixture Of Metals Called The properties of a metal can be modified by mixing it with another substance to form an alloy. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. The traditional way of making alloys was to heat. Most alloys form by melting the elements together. For example, brass is an. An. What Is A Mixture Of Metals Called.
From acamrmicheal.weebly.com
Properties of Mixtures ACA Grade 8 Science What Is A Mixture Of Metals Called Alloys can be formed by substituting one. Mixtures can be solids, liquids, gases, or a combination of states of. The traditional way of making alloys was to heat. A mixture consists of two or more chemically distinct components that do not react with each other. The properties of a metal can be modified by mixing it with another substance to. What Is A Mixture Of Metals Called.
From www.geeksforgeeks.org
What is a Mixture? Definition, Properties, Examples, Types and FAQs What Is A Mixture Of Metals Called Mixtures can be solids, liquids, gases, or a combination of states of. For example, brass is an. A mixture consists of two or more chemically distinct components that do not react with each other. An alloy is a substance made by combining together two or more elements where the primary element is a metal. Alloys are mixtures of metals or. What Is A Mixture Of Metals Called.
From www.slideserve.com
PPT CHAPTER 4 MATERIALS METALS AND NON METALS PowerPoint What Is A Mixture Of Metals Called Most alloys form by melting the elements together. An alloy is a substance made by combining together two or more elements where the primary element is a metal. An alloy may be a solid solution of metal elements (a. The structure of metals explains their high melting and boiling points and their conductivity. Alloys are mixtures of metals or a. What Is A Mixture Of Metals Called.
From sciencedonewright.weebly.com
Unit 2 Classification of Matter Science Done Wright What Is A Mixture Of Metals Called An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. The structure of metals explains their high melting and boiling points and their conductivity. Most alloys form by melting the elements together. For example, brass is an. An alloy is a substance made by combining together two or more elements where. What Is A Mixture Of Metals Called.
From 88guru.com
What Are Homogeneous Mixtures Classification and Applications 88Guru What Is A Mixture Of Metals Called You might find the idea of an alloy as a mixture of metals quite confusing. The structure of metals explains their high melting and boiling points and their conductivity. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. A mixture consists of two or more chemically distinct components that do. What Is A Mixture Of Metals Called.