How Does Surface Tension Affect Adhesion . Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. This general effect is called surface tension. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and boiling point. When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of water within the tube, while adhesion between the water and the glass creates an upward force that maximizes the amount of glass surface in contact with the water. Learn how intermolecular forces, polarity, cohesion, and. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area.
from www.rheologylab.com
Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of water within the tube, while adhesion between the water and the glass creates an upward force that maximizes the amount of glass surface in contact with the water. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. Learn how intermolecular forces, polarity, cohesion, and. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and boiling point. This general effect is called surface tension. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area.
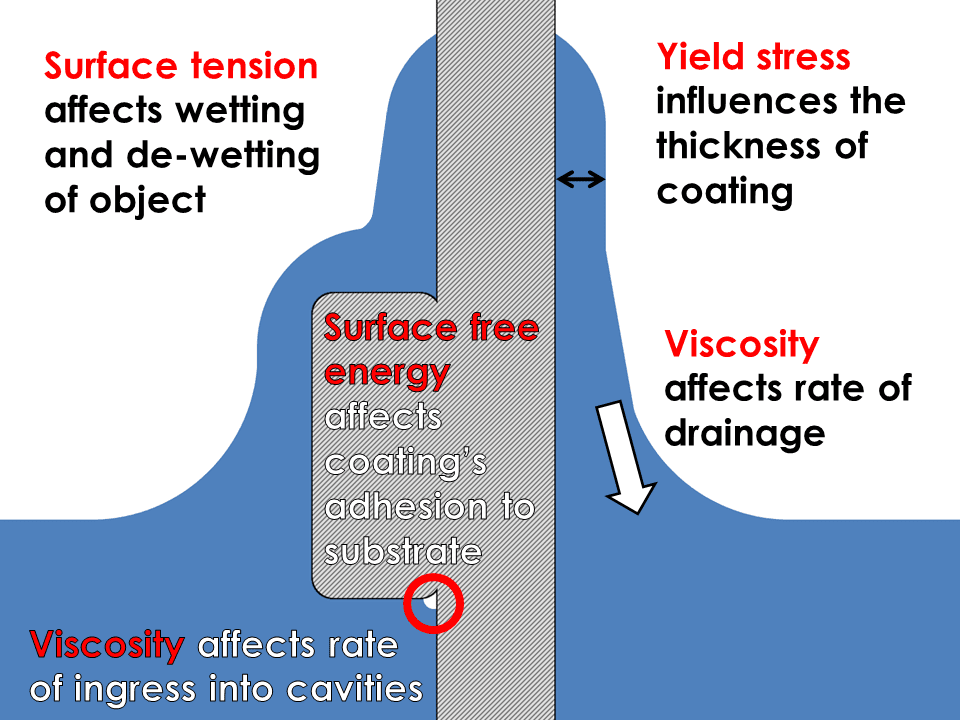
Dip Coating Viscosity, Yield Stress and Surface Tension Rheology Lab
How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and boiling point. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. This general effect is called surface tension. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Learn how intermolecular forces, polarity, cohesion, and. When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of water within the tube, while adhesion between the water and the glass creates an upward force that maximizes the amount of glass surface in contact with the water. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and boiling point.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Learn. How Does Surface Tension Affect Adhesion.
From byjus.com
Explain the surface tension phenomenon with examples. How Does Surface Tension Affect Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and boiling point. Surface tension is the. How Does Surface Tension Affect Adhesion.
From courses.lumenlearning.com
Cohesion and Adhesion in Liquids Surface Tension and Capillary Action How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Learn how. How Does Surface Tension Affect Adhesion.
From www.iqsdirectory.com
Adhesive Tape What Is It? How Is It Made? Uses, Application How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. Learn how intermolecular forces, polarity, cohesion, and. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Surface tension is the force per unit length or energy per unit area that resists stretching. How Does Surface Tension Affect Adhesion.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download How Does Surface Tension Affect Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. This general effect is called surface tension. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary. How Does Surface Tension Affect Adhesion.
From www.slideserve.com
PPT EXAM I Powerpoint II A Little Chemistry PowerPoint Presentation How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. This general effect is called surface tension. Learn how intermolecular forces, polarity, cohesion, and. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. When a glass capillary is put. How Does Surface Tension Affect Adhesion.
From plasticsdecorating.com
Effects of Surface Treatment on Adhesion for Plastic Components How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of water within the tube, while adhesion between the water and the glass creates an upward force that maximizes the amount of. How Does Surface Tension Affect Adhesion.
From onlinelibrary.wiley.com
Understanding Surface Adhesion in Nature A Peeling Model Gu 2016 How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. This general effect is called surface tension. When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of water within the tube, while adhesion between the water and the glass creates an. How Does Surface Tension Affect Adhesion.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint How Does Surface Tension Affect Adhesion Learn how intermolecular forces, polarity, cohesion, and. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Learn how cohesive and adhesive forces affect the properties of liquids, such as. How Does Surface Tension Affect Adhesion.
From pressbooks.online.ucf.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary How Does Surface Tension Affect Adhesion This general effect is called surface tension. Learn how intermolecular forces, polarity, cohesion, and. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and boiling point. Learn how cohesive and. How Does Surface Tension Affect Adhesion.
From www.slideshare.net
Properties of Water PowerPoint, Adhesion, Cohesion, Surface Tension How Does Surface Tension Affect Adhesion When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of water within the tube, while adhesion between the water and the glass creates an upward force that maximizes the amount of glass surface in contact with the water. Cohesive forces between molecules cause the surface of a liquid to contract. How Does Surface Tension Affect Adhesion.
From www.slideserve.com
PPT Just plain old water! PowerPoint Presentation, free download ID How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of. How Does Surface Tension Affect Adhesion.
From www.studypool.com
SOLUTION Cohesion adhesion surface tension Studypool How Does Surface Tension Affect Adhesion Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. Learn how intermolecular forces, polarity, cohesion, and. Learn how cohesive and adhesive forces affect the properties of liquids, such. How Does Surface Tension Affect Adhesion.
From dokumen.tips
(PDF) Cohesion, Surface Tension, and Adhesion DOKUMEN.TIPS How Does Surface Tension Affect Adhesion Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of. How Does Surface Tension Affect Adhesion.
From www.laserax.com
How to Do Surface Preparation for Adhesive Bonding Laserax How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and boiling point. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface.. How Does Surface Tension Affect Adhesion.
From hxexpttsa.blob.core.windows.net
What Do Cohesion Surface Tension And Adhesion at Michael Washington blog How Does Surface Tension Affect Adhesion When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of water within the tube, while adhesion between the water and the glass creates an upward force that maximizes the amount of glass surface in contact with the water. Cohesive forces between molecules cause the surface of a liquid to contract. How Does Surface Tension Affect Adhesion.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID5692577 How Does Surface Tension Affect Adhesion When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of water within the tube, while adhesion between the water and the glass creates an upward force that maximizes the amount of glass surface in contact with the water. Learn how cohesive and adhesive forces affect the properties of liquids, such. How Does Surface Tension Affect Adhesion.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How Does Surface Tension Affect Adhesion When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of water within the tube, while adhesion between the water and the glass creates an upward force that maximizes the amount of glass surface in contact with the water. Learn how cohesive and adhesive forces affect the properties of liquids, such. How Does Surface Tension Affect Adhesion.
From slideplayer.com
Chemical Properties of Water ppt download How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Learn how. How Does Surface Tension Affect Adhesion.
From www.youtube.com
Surface Tension and Adhesion Fluids Physics Khan Academy YouTube How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. When a. How Does Surface Tension Affect Adhesion.
From www.slideserve.com
PPT Cohesion, Adhesion, and Surface Tension PowerPoint Presentation How Does Surface Tension Affect Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of water within the tube, while adhesion between the water and the glass creates an upward force that maximizes the amount of glass. How Does Surface Tension Affect Adhesion.
From ar.inspiredpencil.com
The Relationship Between Hydrogen Bonding And Surface Tension, Adhesion How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. This general effect is called surface tension. Learn how intermolecular forces, polarity, cohesion, and. Cohesive forces between molecules cause the. How Does Surface Tension Affect Adhesion.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint How Does Surface Tension Affect Adhesion Learn how intermolecular forces, polarity, cohesion, and. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Learn how cohesive and adhesive forces affect the properties of liquids, such as. How Does Surface Tension Affect Adhesion.
From www.vrogue.co
Ppt Lesson 3 Cohesion Adhesion Surface Tension Powerp vrogue.co How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Learn. How Does Surface Tension Affect Adhesion.
From www.slideserve.com
PPT Understanding Water PowerPoint Presentation, free download ID How Does Surface Tension Affect Adhesion Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. Learn. How Does Surface Tension Affect Adhesion.
From www.slideserve.com
PPT Adhesives and Adhesion PowerPoint Presentation, free download How Does Surface Tension Affect Adhesion Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Learn how intermolecular forces, polarity, cohesion, and. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Cohesive forces between molecules cause the surface of a liquid to contract to. How Does Surface Tension Affect Adhesion.
From www.sliderbase.com
Adhesion How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of water within the tube,. How Does Surface Tension Affect Adhesion.
From sciencenotes.org
Capillary Action What It Is and How It Works How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Learn how. How Does Surface Tension Affect Adhesion.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint How Does Surface Tension Affect Adhesion Learn how intermolecular forces, polarity, cohesion, and. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and boiling point. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the force per unit length or energy per unit area that resists. How Does Surface Tension Affect Adhesion.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Learn how. How Does Surface Tension Affect Adhesion.
From www.rheologylab.com
Dip Coating Viscosity, Yield Stress and Surface Tension Rheology Lab How Does Surface Tension Affect Adhesion This general effect is called surface tension. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. Learn how intermolecular forces, polarity, cohesion, and. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Learn how cohesive and adhesive. How Does Surface Tension Affect Adhesion.
From slideplayer.com
Basics of Chemistry Biology 9/22/ ppt download How Does Surface Tension Affect Adhesion This general effect is called surface tension. Learn how intermolecular forces, polarity, cohesion, and. When a glass capillary is put into water, the surface tension due to cohesive forces constricts the surface area of water within the tube, while adhesion between the water and the glass creates an upward force that maximizes the amount of glass surface in contact with. How Does Surface Tension Affect Adhesion.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Does Surface Tension Affect Adhesion Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. Learn how intermolecular forces, polarity, cohesion, and. Learn how cohesive and adhesive forces affect the properties of liquids, such. How Does Surface Tension Affect Adhesion.
From www.youtube.com
Chemistry Explained Viscosity, Surface Tension, Adhesion, Cohesion How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and boiling point. Learn how intermolecular forces, polarity, cohesion, and. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest. How Does Surface Tension Affect Adhesion.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint How Does Surface Tension Affect Adhesion Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension and capillary action. Learn how cohesive and adhesive forces affect the properties of liquids, such as surface tension, capillary action, and bubbles. Surface tension is the force per unit length or energy per unit area that resists stretching and rupture of a liquid surface. Cohesive. How Does Surface Tension Affect Adhesion.