Bromide Ion And Chlorine Reaction . When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Cl 2 (aq) + 2nabr(aq) 2nacl(aq). Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. 3 the displacement reaction between chlorine and sodium bromide can be represented by the equation below. The reaction between a c=c double bond and bromine (br2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The bromine reagent is in a reddish color,. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: When bromine is added to the sample, if the reddish color disappear, that means the. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms.
from www.numerade.com
When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: 3 the displacement reaction between chlorine and sodium bromide can be represented by the equation below. Cl 2 (aq) + 2nabr(aq) 2nacl(aq). Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. When bromine is added to the sample, if the reddish color disappear, that means the. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The reaction between a c=c double bond and bromine (br2) can be used as a test for the presence of alkene in an unknown sample. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide.
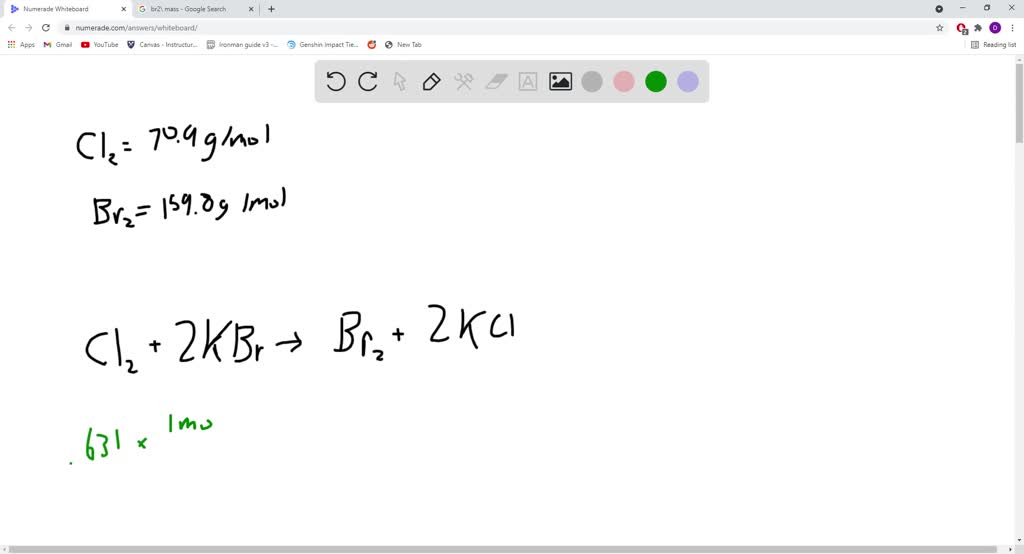
⏩SOLVEDGaseous chlorine will displace bromide ion from an aqueous… Numerade
Bromide Ion And Chlorine Reaction When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The reaction between a c=c double bond and bromine (br2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. 3 the displacement reaction between chlorine and sodium bromide can be represented by the equation below. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The bromine reagent is in a reddish color,. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Cl 2 (aq) + 2nabr(aq) 2nacl(aq). When bromine is added to the sample, if the reddish color disappear, that means the. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon.
From pubs.acs.org
and Mechanism of Bromate−Bromide Reaction Catalyzed by Acetate Chemistry Bromide Ion And Chlorine Reaction The reaction between a c=c double bond and bromine (br2) can be used as a test for the presence of alkene in an unknown sample. When bromine is added to the sample, if the reddish color disappear, that means the. Cl 2 (aq) + 2nabr(aq) 2nacl(aq). Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. Bromide Ion And Chlorine Reaction.
From www.numerade.com
SOLVEDGaseous chlorine will displace bromide ion from an aqueous solution of potassium bromide Bromide Ion And Chlorine Reaction When bromine is added to the sample, if the reddish color disappear, that means the. The bromine reagent is in a reddish color,. Cl 2 (aq) + 2nabr(aq) 2nacl(aq). The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine. Bromide Ion And Chlorine Reaction.
From www.numerade.com
SOLVED Metals bond with halogens to form colorless metal halides. During an experiment Bromide Ion And Chlorine Reaction The bromine reagent is in a reddish color,. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: When bromine is added to the sample, if the reddish. Bromide Ion And Chlorine Reaction.
From www.toppr.com
In potassium bromide solution, chlorine displace Bromide Ion And Chlorine Reaction Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. 3 the displacement reaction between chlorine and sodium bromide can be represented by the equation below.. Bromide Ion And Chlorine Reaction.
From www.numerade.com
SOLVED Write equations for the halfreactions that occur in the electrolysis of molten Bromide Ion And Chlorine Reaction Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. When bromine is added to the sample, if the reddish color disappear, that means the. Cl 2 (aq) +. Bromide Ion And Chlorine Reaction.
From www.reddit.com
How does the negatively charged bromide ion from the heterolytic fission of bromine molecule Bromide Ion And Chlorine Reaction 3 the displacement reaction between chlorine and sodium bromide can be represented by the equation below. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine.. Bromide Ion And Chlorine Reaction.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b)... Download Scientific Diagram Bromide Ion And Chlorine Reaction If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The bromine reagent is in a reddish color,. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. The reaction between a c=c double bond and bromine (br2) can be used as a test. Bromide Ion And Chlorine Reaction.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Bromide Ion And Chlorine Reaction Cl 2 (aq) + 2nabr(aq) 2nacl(aq). 3 the displacement reaction between chlorine and sodium bromide can be represented by the equation below. The bromine reagent is in a reddish color,. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: When bromine is added to the sample, if the reddish color disappear, that. Bromide Ion And Chlorine Reaction.
From www.youtube.com
How to Balance NaBr + Cl2 = NaCl + Br2 (Sodium Bromide + Chlorine gas) YouTube Bromide Ion And Chlorine Reaction Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. 3 the displacement reaction between chlorine and sodium bromide can be represented by the. Bromide Ion And Chlorine Reaction.
From www.bigstockphoto.com
Test Bromides Iodides Image & Photo (Free Trial) Bigstock Bromide Ion And Chlorine Reaction Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The reaction between a c=c double bond and bromine (br2) can be used as a test for the presence of alkene in an unknown. Bromide Ion And Chlorine Reaction.
From socratic.org
What is the balanced chemical equation for this reaction Gaseous chlorine reacts with an Bromide Ion And Chlorine Reaction When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Cl 2 (aq) + 2nabr(aq) 2nacl(aq). If you. Bromide Ion And Chlorine Reaction.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce aqueous sodium chloride and Bromide Ion And Chlorine Reaction When bromine is added to the sample, if the reddish color disappear, that means the. Cl 2 (aq) + 2nabr(aq) 2nacl(aq). If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The bromine reagent is in a reddish color,. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. The. Bromide Ion And Chlorine Reaction.
From www.essentialchemicalindustry.org
Chlorine Bromide Ion And Chlorine Reaction Cl 2 (aq) + 2nabr(aq) 2nacl(aq). The reaction between a c=c double bond and bromine (br2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in a reddish color,. When bromine is added to the sample, if the reddish color disappear, that means the. 3 the displacement reaction between chlorine. Bromide Ion And Chlorine Reaction.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine to bromate and bromide Bromide Ion And Chlorine Reaction Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Cl 2 (aq) + 2nabr(aq) 2nacl(aq). Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms.. Bromide Ion And Chlorine Reaction.
From spmchemistry.blog.onlinetuition.com.my
Examples of Redox Reaction Displacement of Halogen SPM Chemistry Bromide Ion And Chlorine Reaction Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. When bromine is added to. Bromide Ion And Chlorine Reaction.
From www.slideserve.com
PPT Q9 What are the chlorine and bromine reactions that destroy stratospheric ozone Bromide Ion And Chlorine Reaction Cl 2 (aq) + 2nabr(aq) 2nacl(aq). When bromine is added to the sample, if the reddish color disappear, that means the. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Draw the structure of. Bromide Ion And Chlorine Reaction.
From www.youtube.com
Type of Reaction for NaBr + Cl2 = NaCl + Br2 YouTube Bromide Ion And Chlorine Reaction The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Cl 2 (aq) + 2nabr(aq) 2nacl(aq). The reaction between a c=c double bond and bromine (br2) can be used as a test for the presence of alkene in an unknown sample. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction. Bromide Ion And Chlorine Reaction.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Molecules Combine with Bromide Ion And Chlorine Reaction Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Cl 2 (aq) + 2nabr(aq) 2nacl(aq). When alkenes (also known as olefins) are treated. Bromide Ion And Chlorine Reaction.
From www.alamy.com
Bubbling Chlorine Gas into Sodium Bromide to Yield Bromine Stock Photo Alamy Bromide Ion And Chlorine Reaction If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. 3 the displacement reaction between chlorine and sodium bromide can be. Bromide Ion And Chlorine Reaction.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Bromide Ion And Chlorine Reaction If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) +. Bromide Ion And Chlorine Reaction.
From slidetodoc.com
Chemical Equations Reactions Chemistry A Chemical Reactions You Bromide Ion And Chlorine Reaction When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. The bromine reagent is in a reddish color,. When bromine is added to the sample, if the reddish color disappear, that means the. The reaction between a c=c double bond and bromine (br2) can be. Bromide Ion And Chlorine Reaction.
From www.numerade.com
SOLVED Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Bromide Ion And Chlorine Reaction When bromine is added to the sample, if the reddish color disappear, that means the. The reaction between a c=c double bond and bromine (br2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Because chlorine is more reactive than. Bromide Ion And Chlorine Reaction.
From www.youtube.com
TEST FOR HALIDE IONS (Chloride, Bromide and Iodide ions) 3D animation YouTube Bromide Ion And Chlorine Reaction Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. 3 the displacement reaction between chlorine and sodium bromide can be represented by the equation below. The bromine reagent is in a reddish color,. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The reaction between a c=c double bond and bromine. Bromide Ion And Chlorine Reaction.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Bromide Ion And Chlorine Reaction The bromine reagent is in a reddish color,. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: 3 the displacement reaction between chlorine and sodium bromide can be represented by the equation below. When alkenes (also known as. Bromide Ion And Chlorine Reaction.
From www.youtube.com
Balancing Chemical Equations. Part 4 Aluminium bromide and chlorine. YouTube Bromide Ion And Chlorine Reaction Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. When bromine. Bromide Ion And Chlorine Reaction.
From www.numerade.com
SOLVED Potassium bromide + chlorine = potassium chloride + bromine chemical eq..... Bromide Ion And Chlorine Reaction The reaction between a c=c double bond and bromine (br2) can be used as a test for the presence of alkene in an unknown sample. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Cl 2 (aq) + 2nabr(aq) 2nacl(aq). When bromine is added to the sample, if the reddish color disappear,. Bromide Ion And Chlorine Reaction.
From pubs.acs.org
The Multiple Role of Bromide Ion in PPCPs Degradation under UV/Chlorine Treatment Bromide Ion And Chlorine Reaction Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: 3 the displacement reaction between chlorine and sodium bromide can be represented by the equation below. The bromine reagent is in a reddish color,. Draw the structure of the product. Bromide Ion And Chlorine Reaction.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine molecule, and (c) a bromide ion Bromide Ion And Chlorine Reaction The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The reaction between a c=c double bond and bromine (br2) can be used as a test for the presence of alkene in an unknown sample. When bromine is added to the sample, if the reddish color disappear, that means the. When alkenes (also known as olefins). Bromide Ion And Chlorine Reaction.
From www.numerade.com
⏩SOLVEDGaseous chlorine will displace bromide ion from an aqueous… Numerade Bromide Ion And Chlorine Reaction The bromine reagent is in a reddish color,. 3 the displacement reaction between chlorine and sodium bromide can be represented by the equation below. Cl 2 (aq) + 2nabr(aq) 2nacl(aq). Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. When bromine is added to the sample, if the reddish color disappear, that means the. Cl 2. Bromide Ion And Chlorine Reaction.
From fyoebqvdj.blob.core.windows.net
Potassium Bromide And Chlorine Water at Tracy Mendez blog Bromide Ion And Chlorine Reaction 3 the displacement reaction between chlorine and sodium bromide can be represented by the equation below. The bromine reagent is in a reddish color,. Cl 2 (aq) + 2nabr(aq) 2nacl(aq). Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. When bromine is added to the sample, if. Bromide Ion And Chlorine Reaction.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Chloride+Bromine YouTube Bromide Ion And Chlorine Reaction 3 the displacement reaction between chlorine and sodium bromide can be represented by the equation below. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1]. Bromide Ion And Chlorine Reaction.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Bromide Ion And Chlorine Reaction Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. When bromine is added to the sample, if the reddish color disappear, that means the. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The reaction between a c=c double. Bromide Ion And Chlorine Reaction.
From www.youtube.com
Bromide ions with chlorine water YouTube Bromide Ion And Chlorine Reaction When bromine is added to the sample, if the reddish color disappear, that means the. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Draw the structure of the product formed when a given. Bromide Ion And Chlorine Reaction.
From www.youtube.com
Chlorine Water + Sodium Bromide YouTube Bromide Ion And Chlorine Reaction The reaction between a c=c double bond and bromine (br2) can be used as a test for the presence of alkene in an unknown sample. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Cl 2 (aq). Bromide Ion And Chlorine Reaction.
From www.slideshare.net
Oxidation & reduction Bromide Ion And Chlorine Reaction When bromine is added to the sample, if the reddish color disappear, that means the. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The. Bromide Ion And Chlorine Reaction.