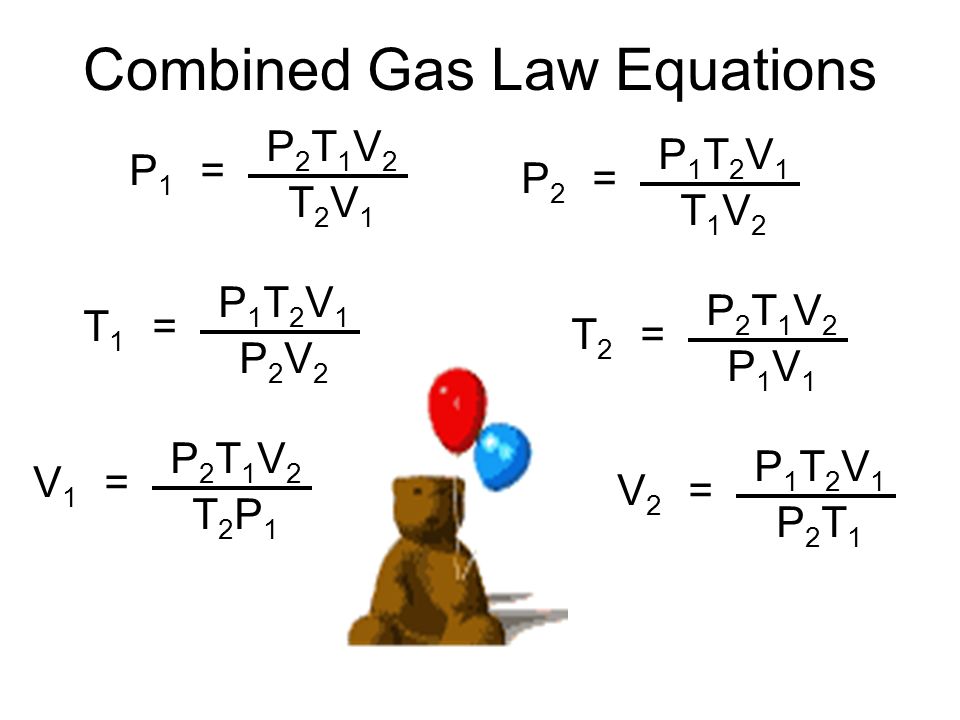
Gas Law Equation . The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. In such a case, all gases obey an equation of state known as the ideal gas law: The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law is very simply expressed: Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and.
from inspiritvr.com
Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. In such a case, all gases obey an equation of state known as the ideal gas law: The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law is very simply expressed: Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and.
Combined Gas Law Study Guide Inspirit Learning Inc
Gas Law Equation Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The ideal gas law is very simply expressed: The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. In such a case, all gases obey an equation of state known as the ideal gas law:
From inspiritvr.com
Combined Gas Law Study Guide Inspirit Learning Inc Gas Law Equation In such a case, all gases obey an equation of state known as the ideal gas law: \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. The ideal gas law is the equation of state for an. Gas Law Equation.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID7067134 Gas Law Equation \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. Although the law describes the behavior of. Gas Law Equation.
From www.britannica.com
perfect gas law chemistry and physics Britannica Gas Law Equation In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law is very simply expressed: Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in. Gas Law Equation.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID3344909 Gas Law Equation In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law is. Gas Law Equation.
From www.slideserve.com
PPT Thermodynamic Properties PowerPoint Presentation, free download ID503896 Gas Law Equation \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The three fundamental gas laws discover the relationship. Gas Law Equation.
From melonyy-upset.blogspot.com
Ideal Gas Law Constant R Values physical chemistry What volume does one mole of an ideal Gas Law Equation \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. In such a case, all gases obey an. Gas Law Equation.
From www.youtube.com
1.3 Ideal gas equation YouTube Gas Law Equation In such a case, all gases obey an equation of state known as the ideal gas law: \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. The ideal gas law is very simply expressed: The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute. Gas Law Equation.
From owlcation.com
The Theories and Behavior of Gas Owlcation Gas Law Equation Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The ideal gas law is very simply expressed: The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount. Gas Law Equation.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Gas Law Equation \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. The ideal gas law is very simply expressed: In such a case, all gases obey an equation of state known as the ideal gas law: Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. The three fundamental. Gas Law Equation.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Gas Law Equation Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. The three fundamental gas laws discover. Gas Law Equation.
From slideplayer.com
AGENDA 10/28/08 DO NOW (5 mins) Solving Gas Law Problem ppt download Gas Law Equation Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. In such a case, all gases obey an equation of state known as the ideal gas law: The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas law, relation between the pressure p, volume. Gas Law Equation.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID3344909 Gas Law Equation Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. The three fundamental gas laws discover. Gas Law Equation.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 Gas Law Equation In such a case, all gases obey an equation of state known as the ideal gas law: \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. The ideal gas law is very simply expressed: The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas law, relation between. Gas Law Equation.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID3252378 Gas Law Equation Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of. Gas Law Equation.
From www.slideserve.com
PPT Introduction to the Gas Laws PowerPoint Presentation, free download ID5824200 Gas Law Equation Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law is. Gas Law Equation.
From www.shutterstock.com
Gay lussac's law 37 images, photos et images vectorielles de stock Shutterstock Gas Law Equation Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The ideal gas law is very simply expressed: Although the law describes the behavior of an ideal gas, it approximates real gas. Gas Law Equation.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Presentation ID6855999 Gas Law Equation The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. In such a case, all gases. Gas Law Equation.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download ID6717792 Gas Law Equation In such a case, all gases obey an equation of state known as the ideal gas law: Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. The ideal gas law is very simply expressed: The ideal gas. Gas Law Equation.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation ID352441 Gas Law Equation Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. In such a case, all gases obey an equation of state known as the ideal gas law: Although the law describes the. Gas Law Equation.
From slideplayer.com
The Behavior of Gases. ppt download Gas Law Equation The ideal gas law is very simply expressed: \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other.. Gas Law Equation.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation ID223578 Gas Law Equation Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. The three fundamental gas. Gas Law Equation.
From pametno21.blogspot.com
V Formula Chemistry pametno Gas Law Equation The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The ideal gas law is the. Gas Law Equation.
From sciencenotes.org
Ideal Gas Law Formula and Examples Gas Law Equation Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. \ [ pv=nrt\] from. Gas Law Equation.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID3344909 Gas Law Equation \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high. Gas Law Equation.
From www.sliderbase.com
Gas Laws Presentation Chemistry Gas Law Equation \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the. Gas Law Equation.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation ID223578 Gas Law Equation \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. In such a case, all gases. Gas Law Equation.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Gas Law Equation Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. In such a case, all gases obey an equation of state known as the ideal gas law: The three fundamental gas laws. Gas Law Equation.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID4187174 Gas Law Equation Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. The three fundamental gas. Gas Law Equation.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID5758540 Gas Law Equation Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Although the law describes the behavior. Gas Law Equation.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4387987 Gas Law Equation The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law is. Gas Law Equation.
From www.slideserve.com
PPT The Combined Gas Law PowerPoint Presentation, free download ID4693587 Gas Law Equation In such a case, all gases obey an equation of state known as the ideal gas law: \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law is very simply expressed: Ideal gas law, relation between. Gas Law Equation.
From millingschem.com
Chemistry Resources 3 MillingsChem Gas Law Equation Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. In such a case, all gases obey an equation of state known as the ideal gas law: The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law is the equation of state. Gas Law Equation.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 Gas Law Equation The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high. Gas Law Equation.
From mmerevise.co.uk
The Ideal Gas Equation MME Gas Law Equation In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. Ideal gas law,. Gas Law Equation.
From www.youtube.com
Gas Law Formulas and Equations College Chemistry Study Guide YouTube Gas Law Equation In such a case, all gases obey an equation of state known as the ideal gas law: Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The three fundamental gas laws. Gas Law Equation.