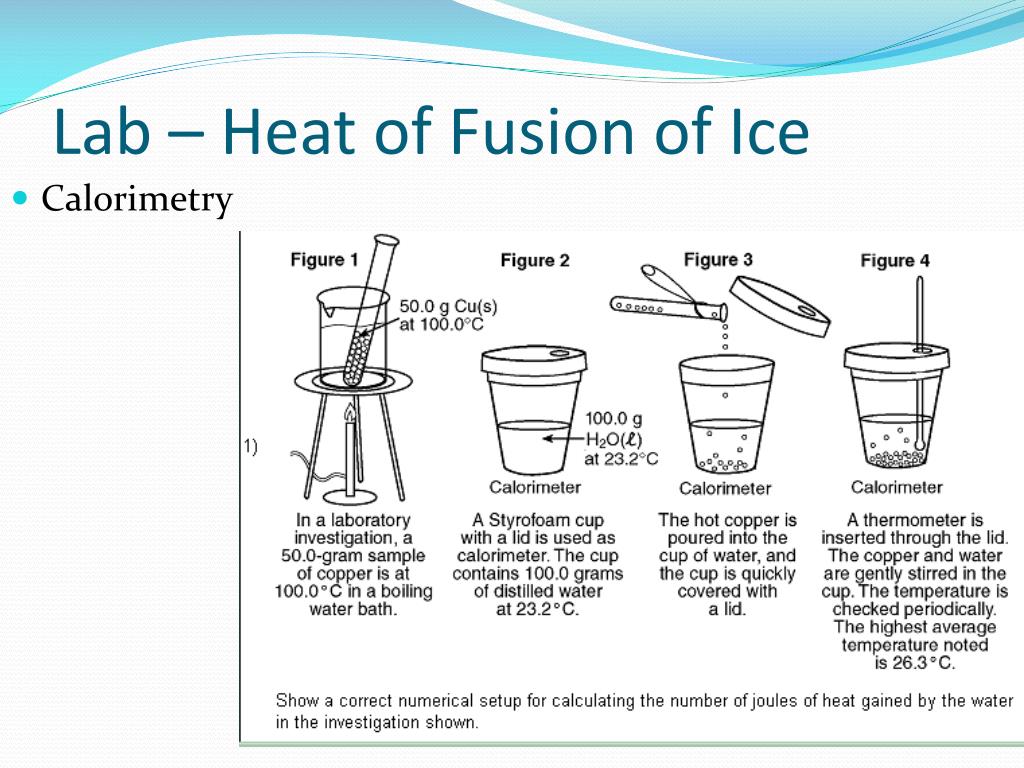
What Is The Value Of Heat Of Fusion Of Ice . The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. Learning how to calculate the. Its units are usually joules per gram (j/g) or calories per gram (cal/g). When studying chemistry, “fusion” simply has the same Learn how to measure heat of fusion for ice. Understand the idea of heat of transformation when ice undergoes a phase change. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. It's also known as enthalpy of fusion. In this experiment, you will determine the energy (in joules) required to melt one gram of ice. You will then determine the molar heat of fusion for ice (in kj/mol).
from www.slideserve.com
Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. Understand the idea of heat of transformation when ice undergoes a phase change. In this experiment, you will determine the energy (in joules) required to melt one gram of ice. You will then determine the molar heat of fusion for ice (in kj/mol). Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. It's also known as enthalpy of fusion. Learning how to calculate the. When studying chemistry, “fusion” simply has the same
PPT Phases of Matter and Solutions PowerPoint Presentation, free download ID2250225
What Is The Value Of Heat Of Fusion Of Ice Understand the idea of heat of transformation when ice undergoes a phase change. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. You will then determine the molar heat of fusion for ice (in kj/mol). The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. It's also known as enthalpy of fusion. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically. Learning how to calculate the. Understand the idea of heat of transformation when ice undergoes a phase change. Learn how to measure heat of fusion for ice. In this experiment, you will determine the energy (in joules) required to melt one gram of ice. When studying chemistry, “fusion” simply has the same Its units are usually joules per gram (j/g) or calories per gram (cal/g).
From www.slideserve.com
PPT Changes in State PowerPoint Presentation, free download ID6995364 What Is The Value Of Heat Of Fusion Of Ice Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. Learning how to calculate the. Its units are usually joules per gram (j/g) or. What Is The Value Of Heat Of Fusion Of Ice.
From study.com
Heat of Fusion Definition, Equation & Examples Video & Lesson Transcript What Is The Value Of Heat Of Fusion Of Ice Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. You will then determine the molar heat of fusion for ice (in kj/mol). It's also known as enthalpy of fusion. Understand the idea of heat of transformation when ice undergoes. What Is The Value Of Heat Of Fusion Of Ice.
From www.slideserve.com
PPT Heat, Temperature and Thermometers PowerPoint Presentation, free download ID3069779 What Is The Value Of Heat Of Fusion Of Ice Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. You will then determine the molar. What Is The Value Of Heat Of Fusion Of Ice.
From studylib.net
Latent Heat of Fusion Experiment What Is The Value Of Heat Of Fusion Of Ice Its units are usually joules per gram (j/g) or calories per gram (cal/g). Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must. What Is The Value Of Heat Of Fusion Of Ice.
From www.slideserve.com
PPT Phases of Matter and Solutions PowerPoint Presentation, free download ID2250225 What Is The Value Of Heat Of Fusion Of Ice Its units are usually joules per gram (j/g) or calories per gram (cal/g). Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically. Learning how to calculate the. Understand the idea of heat of transformation when ice undergoes a phase change. Heat of fusion, also called. What Is The Value Of Heat Of Fusion Of Ice.
From www.slideserve.com
PPT Heat of Fusion PowerPoint Presentation, free download ID2249917 What Is The Value Of Heat Of Fusion Of Ice Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. Its units are usually joules per gram (j/g) or calories per gram (cal/g). Learn how to measure heat of fusion for ice. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy. What Is The Value Of Heat Of Fusion Of Ice.
From slideplayer.com
CHEM 3310 Thermodynamics. ppt download What Is The Value Of Heat Of Fusion Of Ice Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically. Learn how to measure heat of fusion for ice. Learning how to calculate the. Understand the idea of heat of transformation when ice undergoes a phase change. It's also known as enthalpy of fusion. Solids can. What Is The Value Of Heat Of Fusion Of Ice.
From www.slideserve.com
PPT Chapter 12 Thermal Energy PowerPoint Presentation, free download ID4251675 What Is The Value Of Heat Of Fusion Of Ice Learning how to calculate the. You will then determine the molar heat of fusion for ice (in kj/mol). Its units are usually joules per gram (j/g) or calories per gram (cal/g). Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically. It's also known as enthalpy. What Is The Value Of Heat Of Fusion Of Ice.
From www.meritnation.com
The latent heat of fusion of ice is 5 99kjmol at its melting point Then Chemistry What Is The Value Of Heat Of Fusion Of Ice Learn how to measure heat of fusion for ice. In this experiment, you will determine the energy (in joules) required to melt one gram of ice. When studying chemistry, “fusion” simply has the same The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar.. What Is The Value Of Heat Of Fusion Of Ice.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID678389 What Is The Value Of Heat Of Fusion Of Ice You will then determine the molar heat of fusion for ice (in kj/mol). Understand the idea of heat of transformation when ice undergoes a phase change. It's also known as enthalpy of fusion. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. In this experiment, you will determine the. What Is The Value Of Heat Of Fusion Of Ice.
From www.youtube.com
Low Density and High Heat of Fusion of Ice, Chemistry Lecture Sabaq.pk YouTube What Is The Value Of Heat Of Fusion Of Ice Learning how to calculate the. In this experiment, you will determine the energy (in joules) required to melt one gram of ice. Learn how to measure heat of fusion for ice. Its units are usually joules per gram (j/g) or calories per gram (cal/g). You will then determine the molar heat of fusion for ice (in kj/mol). Solids can be. What Is The Value Of Heat Of Fusion Of Ice.
From vdocuments.mx
Melting ice to find the value of the latent heat capacity of fusion of ice [PDF Document] What Is The Value Of Heat Of Fusion Of Ice When studying chemistry, “fusion” simply has the same Learning how to calculate the. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat. What Is The Value Of Heat Of Fusion Of Ice.
From studylib.net
LAB 13 Heat of Fusion of Ice What Is The Value Of Heat Of Fusion Of Ice Its units are usually joules per gram (j/g) or calories per gram (cal/g). In this experiment, you will determine the energy (in joules) required to melt one gram of ice. Learn how to measure heat of fusion for ice. Understand the idea of heat of transformation when ice undergoes a phase change. You will then determine the molar heat of. What Is The Value Of Heat Of Fusion Of Ice.
From www.slideserve.com
PPT Heat and Temperature PowerPoint Presentation, free download ID6031983 What Is The Value Of Heat Of Fusion Of Ice Learn how to measure heat of fusion for ice. Understand the idea of heat of transformation when ice undergoes a phase change. Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically. Solids can be heated to the point where the molecules holding their bonds together. What Is The Value Of Heat Of Fusion Of Ice.
From www.youtube.com
Latent heat of fusion of ice YouTube What Is The Value Of Heat Of Fusion Of Ice When studying chemistry, “fusion” simply has the same Its units are usually joules per gram (j/g) or calories per gram (cal/g). Learn how to measure heat of fusion for ice. In this experiment, you will determine the energy (in joules) required to melt one gram of ice. You will then determine the molar heat of fusion for ice (in kj/mol).. What Is The Value Of Heat Of Fusion Of Ice.
From www.flinnsci.ca
Heat of Fusion of Ice Flinn Scientific What Is The Value Of Heat Of Fusion Of Ice Learn how to measure heat of fusion for ice. Understand the idea of heat of transformation when ice undergoes a phase change. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. The heat which a solid absorbs when it. What Is The Value Of Heat Of Fusion Of Ice.
From studylib.net
Heat of Fusion of Ice What Is The Value Of Heat Of Fusion Of Ice Understand the idea of heat of transformation when ice undergoes a phase change. When studying chemistry, “fusion” simply has the same You will then determine the molar heat of fusion for ice (in kj/mol). Learning how to calculate the. Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a. What Is The Value Of Heat Of Fusion Of Ice.
From www.youtube.com
Latent Heat of Fusion of Ice by an Experiment, Physics Lecture Sabaq.pk YouTube What Is The Value Of Heat Of Fusion Of Ice Understand the idea of heat of transformation when ice undergoes a phase change. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. Learning how to calculate the. Solids can be heated to the point where the molecules holding their bonds together break apart. What Is The Value Of Heat Of Fusion Of Ice.
From mavink.com
Ice Latent Heat What Is The Value Of Heat Of Fusion Of Ice Its units are usually joules per gram (j/g) or calories per gram (cal/g). Learn how to measure heat of fusion for ice. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Understand the idea of heat of transformation when. What Is The Value Of Heat Of Fusion Of Ice.
From www.youtube.com
Calculating Heat of fusion YouTube What Is The Value Of Heat Of Fusion Of Ice Learning how to calculate the. Understand the idea of heat of transformation when ice undergoes a phase change. Its units are usually joules per gram (j/g) or calories per gram (cal/g). Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant. What Is The Value Of Heat Of Fusion Of Ice.
From www.slideserve.com
PPT Heat, Temperature and Thermometers PowerPoint Presentation, free download ID3069779 What Is The Value Of Heat Of Fusion Of Ice In this experiment, you will determine the energy (in joules) required to melt one gram of ice. Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed. What Is The Value Of Heat Of Fusion Of Ice.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk What Is The Value Of Heat Of Fusion Of Ice Learn how to measure heat of fusion for ice. You will then determine the molar heat of fusion for ice (in kj/mol). Learning how to calculate the. Its units are usually joules per gram (j/g) or calories per gram (cal/g). It's also known as enthalpy of fusion. The heat which a solid absorbs when it melts is called the enthalpy. What Is The Value Of Heat Of Fusion Of Ice.
From www.pinterest.com
Heat Of Fusion Easy Science Thermodynamics, Chemical changes, Teaching chemistry What Is The Value Of Heat Of Fusion Of Ice Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. In this experiment, you will determine the energy (in joules) required to melt one gram of ice. You will then determine the molar heat of fusion for ice (in kj/mol).. What Is The Value Of Heat Of Fusion Of Ice.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk What Is The Value Of Heat Of Fusion Of Ice Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. Its units are usually joules per gram (j/g) or calories per gram (cal/g). You will then determine the molar heat of fusion for ice (in kj/mol). In this experiment, you will determine the energy (in joules) required to melt one. What Is The Value Of Heat Of Fusion Of Ice.
From www.slideserve.com
PPT Energy and Chemical Change PowerPoint Presentation ID1278045 What Is The Value Of Heat Of Fusion Of Ice Its units are usually joules per gram (j/g) or calories per gram (cal/g). You will then determine the molar heat of fusion for ice (in kj/mol). Understand the idea of heat of transformation when ice undergoes a phase change. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. When. What Is The Value Of Heat Of Fusion Of Ice.
From studylib.net
To Measure the Specific Latent heat of Fusion of Ice What Is The Value Of Heat Of Fusion Of Ice Learning how to calculate the. It's also known as enthalpy of fusion. When studying chemistry, “fusion” simply has the same Learn how to measure heat of fusion for ice. Its units are usually joules per gram (j/g) or calories per gram (cal/g). Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy. What Is The Value Of Heat Of Fusion Of Ice.
From www.slideserve.com
PPT Fundamentals of Heat Transfer PowerPoint Presentation, free download ID6182043 What Is The Value Of Heat Of Fusion Of Ice You will then determine the molar heat of fusion for ice (in kj/mol). Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Learning how to calculate the. Its units are usually joules per gram (j/g) or calories per gram. What Is The Value Of Heat Of Fusion Of Ice.
From www.ck12.org
Heat of fusion Overview ( Video ) Chemistry CK12 Foundation What Is The Value Of Heat Of Fusion Of Ice The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. In this experiment, you will determine the energy (in joules) required to melt one gram of ice. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of. What Is The Value Of Heat Of Fusion Of Ice.
From byjus.com
49 The amount of heat required to raise the temperature of 75 kg of ice at 0C to water at 10'C What Is The Value Of Heat Of Fusion Of Ice Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically. In this experiment, you will determine the energy (in joules) required to melt one gram of ice. Its units are usually joules per gram (j/g) or calories per gram (cal/g). Learning how to calculate the. You. What Is The Value Of Heat Of Fusion Of Ice.
From www.slideserve.com
PPT Temperature and Heat PowerPoint Presentation, free download ID3080166 What Is The Value Of Heat Of Fusion Of Ice Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically. Understand the idea of heat of transformation when ice undergoes a phase change. In this experiment, you will determine the energy (in joules) required to melt one gram of ice. Learning how to calculate the. It's. What Is The Value Of Heat Of Fusion Of Ice.
From www.youtube.com
Experiment 7 Latent Heat of Fusion of Ice YouTube What Is The Value Of Heat Of Fusion Of Ice Understand the idea of heat of transformation when ice undergoes a phase change. You will then determine the molar heat of fusion for ice (in kj/mol). Its units are usually joules per gram (j/g) or calories per gram (cal/g). Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. Learning. What Is The Value Of Heat Of Fusion Of Ice.
From sciencing.com
How to Measure Heat of Fusion of Ice Sciencing What Is The Value Of Heat Of Fusion Of Ice Learn how to measure heat of fusion for ice. Its units are usually joules per gram (j/g) or calories per gram (cal/g). Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. You will then determine the molar heat of fusion for ice (in kj/mol). Latent heat of fusion, also. What Is The Value Of Heat Of Fusion Of Ice.
From www.youtube.com
heat of fusion of ice notes YouTube What Is The Value Of Heat Of Fusion Of Ice Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. It's also known as enthalpy of fusion. Its units are usually joules per gram (j/g) or calories per gram (cal/g). You will then determine the molar heat of fusion for. What Is The Value Of Heat Of Fusion Of Ice.
From www.youtube.com
QUANTITY OF HEAT SPECIFIC LATENT HEAT OF FUSION OF ICE YouTube What Is The Value Of Heat Of Fusion Of Ice In this experiment, you will determine the energy (in joules) required to melt one gram of ice. Learn how to measure heat of fusion for ice. Learning how to calculate the. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. Understand the idea. What Is The Value Of Heat Of Fusion Of Ice.
From www.ck12.org
Heat of fusion Example 1 ( Video ) Chemistry CK12 Foundation What Is The Value Of Heat Of Fusion Of Ice In this experiment, you will determine the energy (in joules) required to melt one gram of ice. When studying chemistry, “fusion” simply has the same Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. It's also known as enthalpy. What Is The Value Of Heat Of Fusion Of Ice.