Chlorine Formation Reaction . in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: the reaction that takes place is: The ionic equation shows that the chlorine gets both oxidised and. The name given to this is. the reaction that takes place is: this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical.
from stock.adobe.com
formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. the reaction that takes place is: in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. The ionic equation shows that the chlorine gets both oxidised and. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: the reaction that takes place is: The name given to this is.
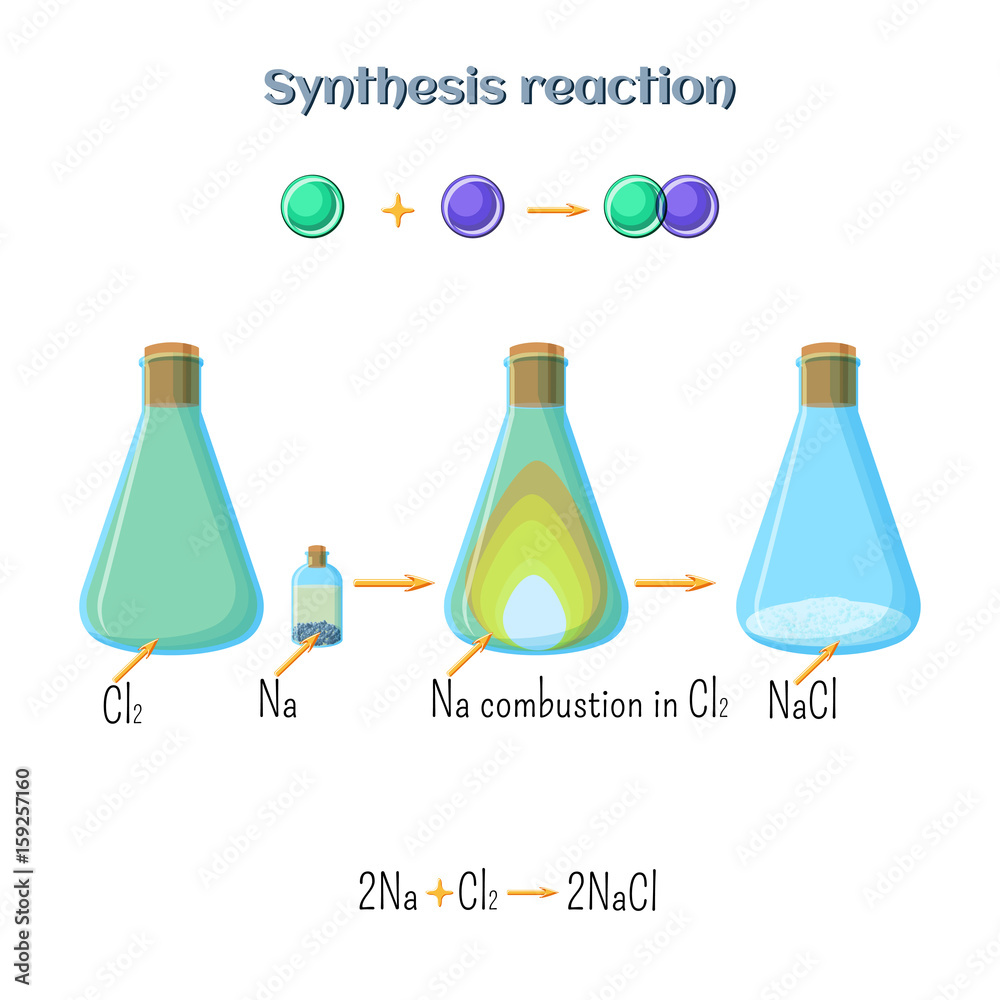
Synthesis reaction sodium chloride formation of sodium metal and
Chlorine Formation Reaction formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. The name given to this is. this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. the reaction that takes place is: formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. The ionic equation shows that the chlorine gets both oxidised and. the reaction that takes place is:
From mavink.com
Ethene And Chlorine Reaction Chlorine Formation Reaction in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. The name given to this is. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq). Chlorine Formation Reaction.
From www.researchgate.net
Potential reaction schemes of phenol during chlorination Download Chlorine Formation Reaction this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: the reaction that takes place is: in order to better understand the mechanism (a detailed look at the step by step process through which. Chlorine Formation Reaction.
From www.slideserve.com
PPT Free Radical Reactions Halogenation of Alkanes PowerPoint Chlorine Formation Reaction the reaction that takes place is: formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. The name given to this is. the reaction that takes place is: The ionic equation shows that the chlorine gets both oxidised and. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) +. Chlorine Formation Reaction.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Chlorine Formation Reaction this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: the reaction that takes place is: formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their. Chlorine Formation Reaction.
From www.chemtube3d.com
Acid Chloride Formation Thionyl Chloride Chlorine Formation Reaction this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. the reaction that takes place is: The ionic equation shows that the chlorine gets both oxidised and. in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine. Chlorine Formation Reaction.
From pubs.acs.org
Chlorination of Amino Acids Reaction Pathways and Reaction Rates Chlorine Formation Reaction The name given to this is. The ionic equation shows that the chlorine gets both oxidised and. the reaction that takes place is: the reaction that takes place is: formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) +. Chlorine Formation Reaction.
From chemistry.stackexchange.com
organic chemistry Formation of acid chlorides Chemistry Stack Exchange Chlorine Formation Reaction formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. the reaction that takes place is: this gives hydrochloric acid (hcl,. Chlorine Formation Reaction.
From www.myxxgirl.com
What Is A Balanced Equation For The Reaction Between Chlorine And My Chlorine Formation Reaction The name given to this is. in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq). Chlorine Formation Reaction.
From www.slideserve.com
PPT Chlorination of Drinking Water PowerPoint Presentation, free Chlorine Formation Reaction the reaction that takes place is: the reaction that takes place is: The ionic equation shows that the chlorine gets both oxidised and. in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. this gives hydrochloric acid (hcl, the inorganic. Chlorine Formation Reaction.
From www.researchgate.net
(PDF) State of the Art of UV/Chlorine Advanced Oxidation Processes Chlorine Formation Reaction the reaction that takes place is: The ionic equation shows that the chlorine gets both oxidised and. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will. Chlorine Formation Reaction.
From csl.noaa.gov
Scientific Assessment of Ozone Depletion 2018 Twenty Questions and Chlorine Formation Reaction The name given to this is. in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. the reaction that takes place is:. Chlorine Formation Reaction.
From www.masterorganicchemistry.com
Thionyl Chloride (SOCl2) Master Organic Chemistry Chlorine Formation Reaction The ionic equation shows that the chlorine gets both oxidised and. formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. The name given to this is. in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we. Chlorine Formation Reaction.
From www.youtube.com
Chlorine and Water AS Chemistry YouTube Chlorine Formation Reaction the reaction that takes place is: this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: in order to better understand the mechanism (a detailed look at the step by step process through which. Chlorine Formation Reaction.
From www.chemistryscl.com
Benzene and Chlorine Reaction C6H6 + Cl2, Mechanism Chlorine Formation Reaction the reaction that takes place is: formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. the reaction that takes place is: The name given to this is. The ionic equation shows that the chlorine gets both oxidised and. this gives hydrochloric acid (hcl, the inorganic product. Chlorine Formation Reaction.
From www.youtube.com
Chloramine Formation and Reactions With Chlorine YouTube Chlorine Formation Reaction this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. The name given to this is. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: the reaction that takes place is: in order to better understand the mechanism (a detailed look at the. Chlorine Formation Reaction.
From www.essentialchemicalindustry.org
Chlorine Chlorine Formation Reaction in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. The name given to this is. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: the reaction that takes place is: The ionic. Chlorine Formation Reaction.
From quizlet.com
Chlorination of Methane Diagram Quizlet Chlorine Formation Reaction this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine. Chlorine Formation Reaction.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes — Master Organic Chemistry Chlorine Formation Reaction in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. The name given to this is. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: the reaction that takes place is: this. Chlorine Formation Reaction.
From pubs.acs.org
Chlorination of Aliphatic Primary Alcohols via Triphosgene Chlorine Formation Reaction this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. the reaction that takes place. Chlorine Formation Reaction.
From www.doubtnut.com
How do you account for formation of ethane during chlorination of meth Chlorine Formation Reaction this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. the reaction that takes place is: 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their. Chlorine Formation Reaction.
From www.youtube.com
AQA Further Reactions of Chlorine YouTube Chlorine Formation Reaction the reaction that takes place is: in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. the reaction that takes place is: The name given. Chlorine Formation Reaction.
From www.researchgate.net
(PDF) Starting computational study of the chlorination mechanism Chlorine Formation Reaction formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. The ionic equation shows that the chlorine gets both oxidised and. this. Chlorine Formation Reaction.
From stock.adobe.com
Synthesis reaction sodium chloride formation of sodium metal and Chlorine Formation Reaction The ionic equation shows that the chlorine gets both oxidised and. the reaction that takes place is: The name given to this is. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: the reaction that takes place is: this gives hydrochloric acid (hcl, the inorganic product of this. Chlorine Formation Reaction.
From encyclopedia.pub
Fundamentals of Chlorine Disinfection Encyclopedia MDPI Chlorine Formation Reaction The name given to this is. in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: formation reactions are chemical reactions that form one. Chlorine Formation Reaction.
From www.sarthaks.com
Select the chain propagation steps in the freeradical chlorination of Chlorine Formation Reaction in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation. Chlorine Formation Reaction.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Chlorine Formation Reaction 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: The name given to this is. The ionic equation shows that the chlorine gets both oxidised and. the reaction that takes place is: the reaction that takes place is: in order to better understand the mechanism (a detailed look. Chlorine Formation Reaction.
From www.toppr.com
In the free radical chlorination of methane, the c Chlorine Formation Reaction The ionic equation shows that the chlorine gets both oxidised and. the reaction that takes place is: this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. the reaction that takes place is: The name given to this is. in order to better understand the mechanism (a detailed look at the. Chlorine Formation Reaction.
From www.youtube.com
13.1.2 Describe the reactions of chlorine and the chlorides referred to Chlorine Formation Reaction The name given to this is. the reaction that takes place is: 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. the reaction that takes place is: The ionic. Chlorine Formation Reaction.
From openchemistryhelp.blogspot.kr
Chemistry Making acid chlorides from carboxylic acids Chlorine Formation Reaction the reaction that takes place is: this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. The ionic equation shows that the chlorine gets both oxidised and. in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine. Chlorine Formation Reaction.
From www.britannica.com
Chemical reaction The BrønstedLowry theory Britannica Chlorine Formation Reaction in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. The name given to this is. formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. this gives hydrochloric acid (hcl, the. Chlorine Formation Reaction.
From www.chemistrystudent.com
Alkanes Free Radical Substitution (ALevel) ChemistryStudent Chlorine Formation Reaction formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o. Chlorine Formation Reaction.
From byjus.com
In the free radical chlorination of methane, the chain initiation step Chlorine Formation Reaction The ionic equation shows that the chlorine gets both oxidised and. 3cl2 (aq) + 6naoh (aq) → 5nacl (aq) + naclo3 (aq) + 3h2o (l) the ionic equation is: this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. the reaction that takes place is: The name given to this is. formation. Chlorine Formation Reaction.
From www.researchgate.net
Important reaction pathways for DBP formation during chlorination and Chlorine Formation Reaction the reaction that takes place is: formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. The name given to this is. the reaction that takes place is: this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. 3cl2 (aq) + 6naoh. Chlorine Formation Reaction.
From ar.inspiredpencil.com
Electrophilic Aromatic Substitution Mechanism Chlorination Chlorine Formation Reaction the reaction that takes place is: this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. the reaction that takes place is: The name given to this is. formation reactions are chemical reactions that form one mole of a substance from its constituent elements in their standard. 3cl2 (aq) + 6naoh. Chlorine Formation Reaction.
From blog.chloramineconsulting.com
Pool Water Chemistry, Part 1 Pool Sanitization Chlorine Formation Reaction this gives hydrochloric acid (hcl, the inorganic product of this reaction) and the methyl radical. the reaction that takes place is: the reaction that takes place is: in order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the. formation reactions. Chlorine Formation Reaction.