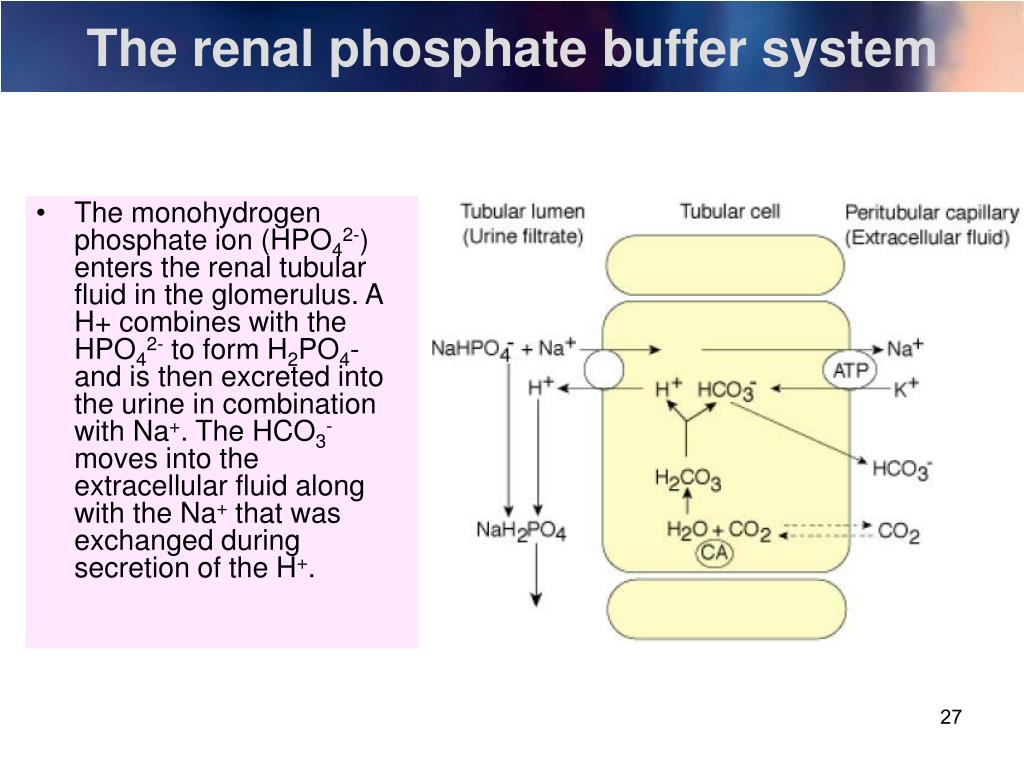
Phosphate Buffer In Blood . Proteins assist with intracellular ph regulation. The concentration of phosphate in the blood is so. If blood ph goes above or below that range, it can. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. The phosphate buffer system is not an important blood buffer as its concentration is too low. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms:
from www.slideserve.com
Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. Proteins assist with intracellular ph regulation. The concentration of phosphate in the blood is so. The phosphate buffer system is not an important blood buffer as its concentration is too low. Phosphates are found in the blood in two forms: If blood ph goes above or below that range, it can. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Phosphates are found in the blood in two forms:
PPT Azin Nowrouzi, PhD PowerPoint Presentation, free download ID
Phosphate Buffer In Blood Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Proteins assist with intracellular ph regulation. The concentration of phosphate in the blood is so. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. The phosphate buffer system is not an important blood buffer as its concentration is too low. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: If blood ph goes above or below that range, it can.
From www.slideserve.com
PPT Azin Nowrouzi, PhD PowerPoint Presentation, free download ID Phosphate Buffer In Blood Proteins assist with intracellular ph regulation. The phosphate buffer system is not an important blood buffer as its concentration is too low. If blood ph goes above or below that range, it can. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphates are found in the blood in two forms: The phosphate. Phosphate Buffer In Blood.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation Phosphate Buffer In Blood Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. If blood ph goes above or below that range, it can. The phosphate buffer system is not an important blood buffer as its concentration is too low. Sodium. Phosphate Buffer In Blood.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner Phosphate Buffer In Blood Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The phosphate buffer system is not an important blood buffer as its concentration is too low. Phosphates are found in the blood in two forms: If blood ph goes above or below that range, it can. The phosphate buffer system, while present globally, is. Phosphate Buffer In Blood.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Phosphate Buffer In Blood The phosphate buffer system, while present globally, is important for the regulation of urine ph. If blood ph goes above or below that range, it can. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. Phosphates are found in the blood in two forms: The concentration of phosphate in the blood is so.. Phosphate Buffer In Blood.
From operaresidences.com.au
Describe the role of the phosphate buffer system? Opera Residences Phosphate Buffer In Blood A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. If blood ph goes above or below that range, it can. Phosphates are found in the blood in two forms: The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. Sodium dihydrogen phosphate (na 2. Phosphate Buffer In Blood.
From www.numerade.com
SOLVED Another buffer found in blood is based on the equilibrium Phosphate Buffer In Blood Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. If blood ph goes above or below that range, it can. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. Phosphates are found in. Phosphate Buffer In Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Phosphate Buffer In Blood The concentration of phosphate in the blood is so. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. Proteins assist with intracellular ph regulation. The phosphate buffer system is not an important blood. Phosphate Buffer In Blood.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer In Blood Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Sodium dihydrogen phosphate (na 2 h. Phosphate Buffer In Blood.
From en.ppt-online.org
Blood biochemistry online presentation Phosphate Buffer In Blood The concentration of phosphate in the blood is so. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: The phosphate buffer system, while present globally, is important for the regulation of urine ph. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen. Phosphate Buffer In Blood.
From www.slideserve.com
PPT AcidBase Equilibrium (Mono and Polyprotic) [Chapter 10,11 Phosphate Buffer In Blood Phosphates are found in the blood in two forms: The phosphate buffer system, while present globally, is important for the regulation of urine ph. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body.. Phosphate Buffer In Blood.
From ppt-online.org
Disorders of metabolism. (Subject 9) презентация онлайн Phosphate Buffer In Blood Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. If blood ph goes above or below that range, it can. The phosphate buffer system is not an important blood buffer as its concentration is too low. Phosphates are found in the blood in two forms: Proteins assist with intracellular ph regulation. Sodium dihydrogen phosphate (na 2 h 2. Phosphate Buffer In Blood.
From labpedia.net
Phosphorus (P), Phosphate (PO4), Phosphorus Phosphate Buffer In Blood Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. Phosphates are found in. Phosphate Buffer In Blood.
From www.slideserve.com
PPT Blood Buffers PowerPoint Presentation, free download ID5687657 Phosphate Buffer In Blood Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Sodium dihydrogen phosphate (na 2. Phosphate Buffer In Blood.
From www.slideserve.com
PPT Teaching Buffering by Comparing Observed and Expected PowerPoint Phosphate Buffer In Blood The concentration of phosphate in the blood is so. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphate Buffer In Blood.
From slideplayer.com
CHAPTER 9 Chemistry ppt download Phosphate Buffer In Blood Proteins assist with intracellular ph regulation. The phosphate buffer system, while present globally, is important for the regulation of urine ph. The concentration of phosphate in the blood is so. The phosphate buffer system is not an important blood buffer as its concentration is too low. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak. Phosphate Buffer In Blood.
From www.slideserve.com
PPT Acid and Base Balance PowerPoint Presentation, free download ID Phosphate Buffer In Blood The phosphate buffer system is not an important blood buffer as its concentration is too low. Phosphates are found in the blood in two forms: If blood ph goes above or below that range, it can. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Sodium dihydrogen phosphate (na 2 h 2 po 4. Phosphate Buffer In Blood.
From www.slideserve.com
PPT Acid and Base Balance PowerPoint Presentation, free download ID Phosphate Buffer In Blood Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphates are found in the blood in two forms: The ph of the blood is maintained between 7.35 and 7.45 by buffers in the. Phosphate Buffer In Blood.
From slideplayer.com
Acid base balance Dr. S. Parthasarathy MD., DA., DNB, MD (Acu), Dip Phosphate Buffer In Blood Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The concentration of phosphate in the blood is so. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. Phosphate Buffer In Blood.
From doctorlib.info
Acidbase Balance Physiology An Illustrated Review Phosphate Buffer In Blood Phosphates are found in the blood in two forms: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Proteins assist with intracellular ph regulation. The concentration of phosphate in the blood is so. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. The. Phosphate Buffer In Blood.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer In Blood Phosphates are found in the blood in two forms: The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. The concentration of phosphate in the blood is so. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. Phosphate Buffer In Blood.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer In Blood Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Phosphates are found in the blood in two forms: The ph of the blood is maintained between 7.35 and 7.45 by buffers in the. Phosphate Buffer In Blood.
From pressbooks.bccampus.ca
26.4 AcidBase Balance Douglas College Human Anatomy and Physiology Phosphate Buffer In Blood If blood ph goes above or below that range, it can. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. Proteins assist with intracellular ph regulation. Sodium. Phosphate Buffer In Blood.
From www.youtube.com
Buffer action in the blood YouTube Phosphate Buffer In Blood If blood ph goes above or below that range, it can. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. A buffer is a solution that can resist ph change upon the addition of an acidic or. Phosphate Buffer In Blood.
From www.youtube.com
Blood Buffers Bicarbonate buffer system Phosphate buffer Protein Phosphate Buffer In Blood Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphates are found in the blood in two forms: The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. Sodium dihydrogen phosphate (na 2 h 2 po 4 −),. Phosphate Buffer In Blood.
From ar.inspiredpencil.com
Phosphate Buffer System Equation Phosphate Buffer In Blood Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. The phosphate buffer system is not an important blood buffer as its concentration is too low. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the. Phosphate Buffer In Blood.
From www.pinterest.com
Physiology Blood Buffer System Behrouz Human body facts Phosphate Buffer In Blood The phosphate buffer system is not an important blood buffer as its concentration is too low. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphates are found in the blood in two forms: The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. Sodium dihydrogen phosphate. Phosphate Buffer In Blood.
From www.researchgate.net
4 questions with answers in BUFFERS Science topic Phosphate Buffer In Blood The concentration of phosphate in the blood is so. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. The phosphate buffer system, while present globally, is important for the regulation. Phosphate Buffer In Blood.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Phosphate Buffer In Blood The phosphate buffer system, while present globally, is important for the regulation of urine ph. Phosphates are found in the blood in two forms: The phosphate buffer system is not an important blood buffer as its concentration is too low. Phosphates are found in the blood in two forms: The concentration of phosphate in the blood is so. Sodium dihydrogen. Phosphate Buffer In Blood.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download Phosphate Buffer In Blood Phosphates are found in the blood in two forms: The concentration of phosphate in the blood is so. If blood ph goes above or below that range, it can. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the. Phosphate Buffer In Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Phosphate Buffer In Blood Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: The phosphate buffer system, while present globally, is important for the regulation of urine ph. Proteins assist with intracellular ph regulation. Sodium dihydrogen phosphate (na 2 h 2 po 4 −),. Phosphate Buffer In Blood.
From www.slideserve.com
PPT Blood Buffers PowerPoint Presentation, free download ID5687657 Phosphate Buffer In Blood Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Proteins assist with intracellular ph regulation. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. If blood ph goes above or below that range, it can. Phosphates are found in the blood in. Phosphate Buffer In Blood.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer In Blood Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: Proteins assist with intracellular ph regulation. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. The ph of the blood is maintained between 7.35. Phosphate Buffer In Blood.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and Phosphate Buffer In Blood Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. The phosphate buffer system, while present globally, is important for the regulation of. Phosphate Buffer In Blood.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Phosphate Buffer In Blood Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Proteins assist with intracellular ph regulation. Phosphates are found in the blood in two forms: The ph of the blood. Phosphate Buffer In Blood.
From derangedphysiology.com
Buffering in acute respiratory acidbase disturbances Deranged Physiology Phosphate Buffer In Blood The phosphate buffer system, while present globally, is important for the regulation of urine ph. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2. Phosphate Buffer In Blood.