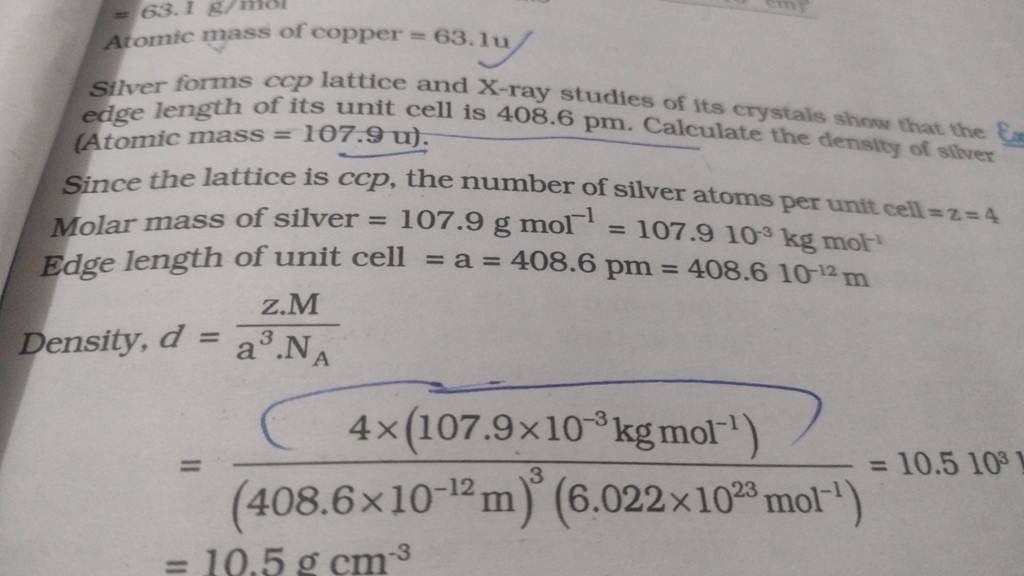Atomic Mass Of Copper 63 And 65 . The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. As you know, the average atomic mass of an element is determined. it has an atomic weight of 63.546 and a mass number of 63. In addition, 27 radioisotopes are known. 65 cu or 6529 cu mass number a: copper metal has two naturally occurring isotopes: a naturally occuring sample of copper consists of 69.2% of 63 c u and 30.8% of 65 c u. copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. 65 (= number of nucleons).
from askfilo.com
65 (= number of nucleons). In addition, 27 radioisotopes are known. 65 cu or 6529 cu mass number a: The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. it has an atomic weight of 63.546 and a mass number of 63. copper metal has two naturally occurring isotopes: a naturally occuring sample of copper consists of 69.2% of 63 c u and 30.8% of 65 c u. copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. As you know, the average atomic mass of an element is determined.
Atomic mass of copper =63.1uSilver forms ccp lattice and Xray studies o..
Atomic Mass Of Copper 63 And 65 The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. As you know, the average atomic mass of an element is determined. 65 (= number of nucleons). In addition, 27 radioisotopes are known. a naturally occuring sample of copper consists of 69.2% of 63 c u and 30.8% of 65 c u. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. it has an atomic weight of 63.546 and a mass number of 63. copper metal has two naturally occurring isotopes: 65 cu or 6529 cu mass number a:
From askfilo.com
Copper has two naturally occurring isotopes 63Cu and 65Cu. If the average.. Atomic Mass Of Copper 63 And 65 As you know, the average atomic mass of an element is determined. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. 65 cu or 6529 cu mass number a: copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the. Atomic Mass Of Copper 63 And 65.
From www.numerade.com
Calculate the relative atomic mass of copper with the following Atomic Mass Of Copper 63 And 65 As you know, the average atomic mass of an element is determined. copper metal has two naturally occurring isotopes: In addition, 27 radioisotopes are known. 65 cu or 6529 cu mass number a: 65 (= number of nucleons). it has an atomic weight of 63.546 and a mass number of 63. copper has two isotopes, 63 cu. Atomic Mass Of Copper 63 And 65.
From techiescientist.com
Is Copper an Element? Techiescientist Atomic Mass Of Copper 63 And 65 copper metal has two naturally occurring isotopes: As you know, the average atomic mass of an element is determined. 65 cu or 6529 cu mass number a: it has an atomic weight of 63.546 and a mass number of 63. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28. Atomic Mass Of Copper 63 And 65.
From www.slideshare.net
Atomic Structure and the Periodic Table Atomic Mass Of Copper 63 And 65 The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. 65 cu or 6529 cu mass number a: In addition, 27 radioisotopes are known. it has an atomic weight of 63.546 and a. Atomic Mass Of Copper 63 And 65.
From www.numerade.com
SOLVED Copper has two isotopes, Cu63 and Cu65. The table below shows Atomic Mass Of Copper 63 And 65 copper metal has two naturally occurring isotopes: it has an atomic weight of 63.546 and a mass number of 63. In addition, 27 radioisotopes are known. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu. Atomic Mass Of Copper 63 And 65.
From www.gauthmath.com
Solved Calculate the relative atomic mass of copper that is composed Atomic Mass Of Copper 63 And 65 As you know, the average atomic mass of an element is determined. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. In addition, 27 radioisotopes are known. 65 (= number of nucleons). The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. . Atomic Mass Of Copper 63 And 65.
From www.chegg.com
Solved 1. Estimate the atomic mass of copper. Copper has two Atomic Mass Of Copper 63 And 65 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. copper metal has two naturally occurring isotopes: a naturally occuring sample of copper consists of 69.2% of 63 c u and 30.8% of 65 c u. In addition, 27 radioisotopes are known. The relative mass of 63 c u. Atomic Mass Of Copper 63 And 65.
From slideplayer.com
ATOMIC STRUCTURE. ppt download Atomic Mass Of Copper 63 And 65 As you know, the average atomic mass of an element is determined. In addition, 27 radioisotopes are known. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole. Atomic Mass Of Copper 63 And 65.
From www.slideserve.com
PPT Warmup PowerPoint Presentation, free download ID5435322 Atomic Mass Of Copper 63 And 65 In addition, 27 radioisotopes are known. 65 cu or 6529 cu mass number a: it has an atomic weight of 63.546 and a mass number of 63. 65 (= number of nucleons). As you know, the average atomic mass of an element is determined. copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass. Atomic Mass Of Copper 63 And 65.
From www.youtube.com
Calculating The Relative Atomic Mass of Copper GCSE Chemistry Atomic Mass Of Copper 63 And 65 copper metal has two naturally occurring isotopes: In addition, 27 radioisotopes are known. As you know, the average atomic mass of an element is determined. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. a naturally occuring sample of copper consists of 69.2% of 63 c u and. Atomic Mass Of Copper 63 And 65.
From www.numerade.com
SOLVED The two naturally occurring isotopes of copper (chemical symbol Atomic Mass Of Copper 63 And 65 In addition, 27 radioisotopes are known. copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. 60 rows copper (29. Atomic Mass Of Copper 63 And 65.
From www.slideserve.com
PPT ATOMIC MASS & AVERAGE ATOMIC MASS PowerPoint Presentation ID Atomic Mass Of Copper 63 And 65 copper metal has two naturally occurring isotopes: 65 cu or 6529 cu mass number a: it has an atomic weight of 63.546 and a mass number of 63. In addition, 27 radioisotopes are known. copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions. Atomic Mass Of Copper 63 And 65.
From www.bartleby.com
Answered Copper has two naturally occurring… bartleby Atomic Mass Of Copper 63 And 65 The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. a naturally occuring sample of copper consists of 69.2% of 63 c u and 30.8% of 65 c u. copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole. Atomic Mass Of Copper 63 And 65.
From www.numerade.com
SOLVED '8 Naturally occurring copper exists in two isotopic forms Atomic Mass Of Copper 63 And 65 a naturally occuring sample of copper consists of 69.2% of 63 c u and 30.8% of 65 c u. As you know, the average atomic mass of an element is determined. 65 (= number of nucleons). copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole. Atomic Mass Of Copper 63 And 65.
From www.slideserve.com
PPT What is atomic mass? PowerPoint Presentation, free download ID Atomic Mass Of Copper 63 And 65 As you know, the average atomic mass of an element is determined. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. copper metal has two naturally occurring isotopes: a naturally occuring sample of copper consists of 69.2% of 63 c u and 30.8% of 65 c u. In. Atomic Mass Of Copper 63 And 65.
From www.numerade.com
SOLVED 3a. What is the average atomic mass of copper on the periodic Atomic Mass Of Copper 63 And 65 The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. copper metal has two naturally occurring isotopes: 65 cu or 6529 cu mass number a: a naturally occuring sample of copper consists of 69.2% of 63 c u and 30.8% of 65 c u. 60 rows copper (29 cu) has. Atomic Mass Of Copper 63 And 65.
From www.chegg.com
Solved The isotopes of copper are^63 Cu and^65 Cu. The Atomic Mass Of Copper 63 And 65 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. copper metal has two naturally occurring isotopes: copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Atomic Mass Of Copper 63 And 65.
From periodictableguide.com
Copper (Cu) Periodic Table (Element Information & More) Atomic Mass Of Copper 63 And 65 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. it has an atomic weight of 63.546 and a mass number of 63. copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and. Atomic Mass Of Copper 63 And 65.
From www.chegg.com
Solved 2. Copper has two isotopes, copper63 and copper65. Atomic Mass Of Copper 63 And 65 copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. The relative mass of 63 c u is 62.93 and that. Atomic Mass Of Copper 63 And 65.
From fyoilezqj.blob.core.windows.net
Copper Atomic Number And Mass Number at Maryjane Anthony blog Atomic Mass Of Copper 63 And 65 65 (= number of nucleons). it has an atomic weight of 63.546 and a mass number of 63. The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. copper metal has two naturally occurring isotopes: As you know, the average atomic mass of an element is determined. In addition, 27 radioisotopes. Atomic Mass Of Copper 63 And 65.
From www.slideserve.com
PPT ISOTOPES PowerPoint Presentation, free download ID5762080 Atomic Mass Of Copper 63 And 65 The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. a naturally occuring sample of copper consists of 69.2% of 63 c u and 30.8% of 65 c u. copper has two. Atomic Mass Of Copper 63 And 65.
From www.vrogue.co
The Average Atomic Mass Of Copper Is 63 546 Amu Natur vrogue.co Atomic Mass Of Copper 63 And 65 The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. In addition, 27 radioisotopes are known. As you know, the average atomic mass of an element is determined. copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are. Atomic Mass Of Copper 63 And 65.
From www.slideshare.net
2 Atomic Structure Atomic Mass Of Copper 63 And 65 The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. As you know, the average atomic mass of an element is determined. it has an atomic weight of 63.546 and a mass number. Atomic Mass Of Copper 63 And 65.
From giokgfwwe.blob.core.windows.net
Copper63 Atomic Number at Linda Edwards blog Atomic Mass Of Copper 63 And 65 copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. 65 (= number of nucleons). it has an atomic weight. Atomic Mass Of Copper 63 And 65.
From www.chegg.com
Solved 1. Estimate the atomic mass of copper. Copper has two Atomic Mass Of Copper 63 And 65 In addition, 27 radioisotopes are known. it has an atomic weight of 63.546 and a mass number of 63. a naturally occuring sample of copper consists of 69.2% of 63 c u and 30.8% of 65 c u. 65 (= number of nucleons). 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu,. Atomic Mass Of Copper 63 And 65.
From www.chegg.com
Solved 1.) Calculate the atomic mass of copperiff Cn63 is Atomic Mass Of Copper 63 And 65 In addition, 27 radioisotopes are known. copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. copper metal has two. Atomic Mass Of Copper 63 And 65.
From slideplayer.com
Unit 2 Atomic Theory & Structure ppt download Atomic Mass Of Copper 63 And 65 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. 65 (= number of nucleons). As you know, the average atomic mass of an element is determined. copper metal has two naturally occurring isotopes: The relative mass of 63 c u is 62.93 and that of 65 c u is. Atomic Mass Of Copper 63 And 65.
From askfilo.com
Atomic mass of copper =63.1uSilver forms ccp lattice and Xray studies o.. Atomic Mass Of Copper 63 And 65 it has an atomic weight of 63.546 and a mass number of 63. 65 (= number of nucleons). copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is. Atomic Mass Of Copper 63 And 65.
From www.meritnation.com
Two isotopes of Copper, 63Cu has a mass of 62 930 u, and 65Cu has a Atomic Mass Of Copper 63 And 65 copper metal has two naturally occurring isotopes: As you know, the average atomic mass of an element is determined. 65 cu or 6529 cu mass number a: it has an atomic weight of 63.546 and a mass number of 63. In addition, 27 radioisotopes are known. 60 rows copper (29 cu) has two stable isotopes, 63 cu. Atomic Mass Of Copper 63 And 65.
From www.coursehero.com
[Solved] What is the atomic mass of copper with isotopes copper63 with Atomic Mass Of Copper 63 And 65 copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu. 60 rows copper (29 cu) has two stable isotopes, 63. Atomic Mass Of Copper 63 And 65.
From periodictable.me
Printable Periodic Table With Atomic Number Atomic Mass Of Copper 63 And 65 The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. In addition, 27 radioisotopes are known. 65 (= number of nucleons). As you know, the average atomic mass of an element is determined. copper metal has two naturally occurring isotopes: a naturally occuring sample of copper consists of 69.2% of 63. Atomic Mass Of Copper 63 And 65.
From ecurrencythailand.com
What Is The Atomic Mass Of Copper 63 And Copper 65? All Answers Atomic Mass Of Copper 63 And 65 As you know, the average atomic mass of an element is determined. copper metal has two naturally occurring isotopes: copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there. Atomic Mass Of Copper 63 And 65.
From www.numerade.com
⏩SOLVEDCalculate Copper has two isotopes C u63 (abundance =69.2 Atomic Mass Of Copper 63 And 65 As you know, the average atomic mass of an element is determined. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. 65 (= number of nucleons). copper metal has two naturally occurring isotopes: a naturally occuring sample of copper consists of 69.2% of 63 c u and 30.8%. Atomic Mass Of Copper 63 And 65.
From www.toppr.com
The average atomic mass of copper is 63.546 amu. Natural copper Atomic Mass Of Copper 63 And 65 it has an atomic weight of 63.546 and a mass number of 63. In addition, 27 radioisotopes are known. The relative mass of 63 c u is 62.93 and that of 65 c u is 64.93. 65 cu or 6529 cu mass number a: copper metal has two naturally occurring isotopes: a naturally occuring sample of copper. Atomic Mass Of Copper 63 And 65.
From www.vrogue.co
The Average Atomic Mass Of Copper Is 63 546 Amu Natur vrogue.co Atomic Mass Of Copper 63 And 65 In addition, 27 radioisotopes are known. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. As you know, the average atomic mass of an element is determined. copper metal has two naturally occurring isotopes: a naturally occuring sample of copper consists of 69.2% of 63 c u and. Atomic Mass Of Copper 63 And 65.