Clock Reaction Order . in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. It should be emphasized that m and. the overall reaction order is the sum of m and n. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. In this experiment, we will examine the effects of both temperature (cold. N are experimentally determined and are not. The good news from equation 5 is that the rate. If the extent of the reaction is. The basic reaction involves hydrogen.
from www.chegg.com
in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. If the extent of the reaction is. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. In this experiment, we will examine the effects of both temperature (cold. It should be emphasized that m and. the overall reaction order is the sum of m and n. N are experimentally determined and are not. The good news from equation 5 is that the rate. The basic reaction involves hydrogen.
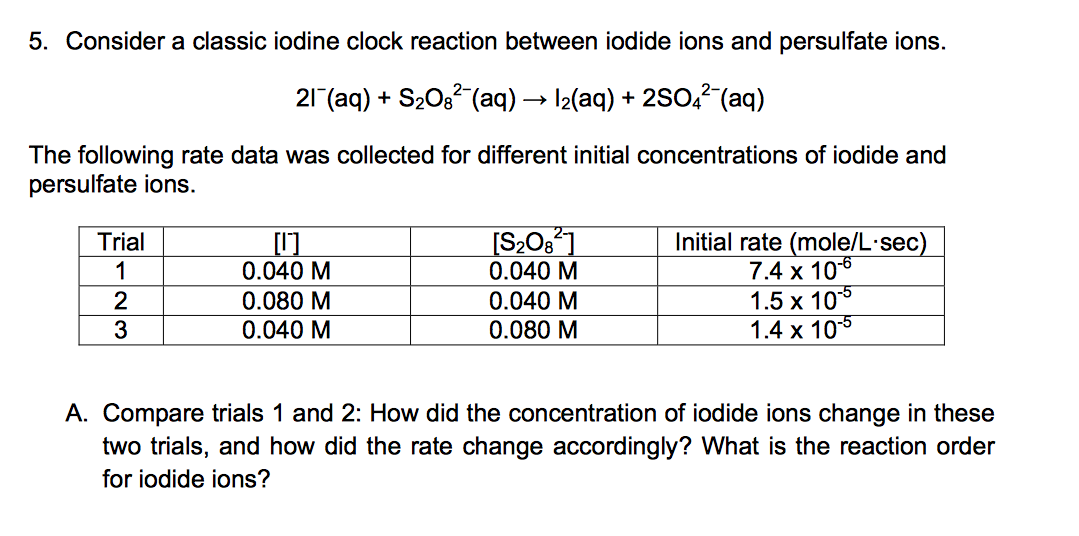
Solved 5. Consider a classic iodine clock reaction between
Clock Reaction Order The basic reaction involves hydrogen. If the extent of the reaction is. In this experiment, we will examine the effects of both temperature (cold. The good news from equation 5 is that the rate. N are experimentally determined and are not. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. the overall reaction order is the sum of m and n. It should be emphasized that m and. The basic reaction involves hydrogen. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction.
From www.youtube.com
Iodine Clock Reaction Chemistry YouTube Clock Reaction Order that is, the reaction is first order with respect to both hydrogen peroxide and iodide. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. N are experimentally determined and are not. the overall reaction order is the sum of m and n. in this experiment we use. Clock Reaction Order.
From www.youtube.com
Clock Reactions Alevel Chemistry OCR, AQA, Edexcel YouTube Clock Reaction Order It should be emphasized that m and. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. In this experiment, we will examine the effects of both temperature (cold. the overall reaction order is. Clock Reaction Order.
From www.slideserve.com
PPT The Amazing Chemical Clock Reaction! PowerPoint Presentation Clock Reaction Order In this experiment, we will examine the effects of both temperature (cold. The basic reaction involves hydrogen. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. The good news from equation 5 is that the rate. that is, the reaction is first order with respect to both hydrogen peroxide. Clock Reaction Order.
From www.savemyexams.com
Iodine Clock Reaction AQA A Level Chemistry Revision Notes 2017 Clock Reaction Order In this experiment, we will examine the effects of both temperature (cold. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. If the extent of the reaction is. the overall reaction order is the sum of m and n. The good news from equation 5 is that the rate.. Clock Reaction Order.
From www.wikihow.com
How to Perform the Iodine Clock Reaction 11 Steps (with Pictures) Clock Reaction Order It should be emphasized that m and. The good news from equation 5 is that the rate. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. If the extent of the reaction is. N are experimentally determined and are not. The basic reaction. Clock Reaction Order.
From www.chegg.com
Solved 5. Consider a classic iodine clock reaction between Clock Reaction Order In this experiment, we will examine the effects of both temperature (cold. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. N are experimentally determined and are not. The basic reaction involves hydrogen. The good news from equation 5 is that the rate.. Clock Reaction Order.
From www.slideserve.com
PPT Reaction order PowerPoint Presentation, free download ID834824 Clock Reaction Order N are experimentally determined and are not. The good news from equation 5 is that the rate. The basic reaction involves hydrogen. It should be emphasized that m and. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. In this experiment, we will examine the effects of both temperature (cold. the overall. Clock Reaction Order.
From www.slideserve.com
PPT Chemical Lab The formaldehyde clock reaction PowerPoint Clock Reaction Order in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. The basic reaction involves hydrogen. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. the overall reaction order is the sum of m. Clock Reaction Order.
From studylib.net
Rates of Chemical Reactions I A Clock Reaction Clock Reaction Order It should be emphasized that m and. In this experiment, we will examine the effects of both temperature (cold. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order. Clock Reaction Order.
From www.slideserve.com
PPT Calculating the Results for Using the Iodine Clock Method to Find Clock Reaction Order The good news from equation 5 is that the rate. the overall reaction order is the sum of m and n. If the extent of the reaction is. It should be emphasized that m and. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. The basic reaction involves hydrogen.. Clock Reaction Order.
From studylib.net
Reaction The Iodine Clock Reaction Clock Reaction Order The basic reaction involves hydrogen. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. In this experiment, we will examine the effects of both temperature (cold. The good news from equation 5 is that the rate. in this experiment we use the initial rate method to find the order. Clock Reaction Order.
From www.slideserve.com
PPT Calculating the Results for Using the Iodine Clock Method to Find Clock Reaction Order The good news from equation 5 is that the rate. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. in this experiment we use the initial rate method to find the order of. Clock Reaction Order.
From studylib.net
Clock ReactionOrders of a Reaction Clock Reaction Order in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. that is, the reaction is first order with respect to both hydrogen peroxide and. Clock Reaction Order.
From www.slideserve.com
PPT Chemical Lab The formaldehyde clock reaction PowerPoint Clock Reaction Order in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order. Clock Reaction Order.
From crunchchemistry.co.uk
What is a clock reaction? Crunch Chemistry Clock Reaction Order The good news from equation 5 is that the rate. the overall reaction order is the sum of m and n. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. N are experimentally determined and are not. In this experiment, we will. Clock Reaction Order.
From www.flinnsci.ca
Iodine Clock Reaction Flinn Scientific Clock Reaction Order in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. N are experimentally determined and are not. In this experiment, we will examine the effects of both temperature (cold. The good news from equation 5 is that the rate. It should be emphasized that. Clock Reaction Order.
From crunchchemistry.co.uk
What is a clock reaction? Crunch Chemistry Clock Reaction Order If the extent of the reaction is. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. N are experimentally determined and are not. The good news from equation 5 is that the rate. In this experiment, we will examine the effects of both temperature (cold. in this experiment we use the initial. Clock Reaction Order.
From www.youtube.com
of a Clock Reaction practice calculations YouTube Clock Reaction Order If the extent of the reaction is. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. N are experimentally determined and are not. The good news from. Clock Reaction Order.
From www.youtube.com
Iodine Clock Reaction Information YouTube Clock Reaction Order The good news from equation 5 is that the rate. the overall reaction order is the sum of m and n. In this experiment, we will examine the effects of both temperature (cold. If the extent of the reaction is. The basic reaction involves hydrogen. It should be emphasized that m and. N are experimentally determined and are not.. Clock Reaction Order.
From www.youtube.com
Iodine Clock Reaction YouTube Clock Reaction Order In this experiment, we will examine the effects of both temperature (cold. The good news from equation 5 is that the rate. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. If the extent of the reaction is. that is, the reaction is first order with respect to both. Clock Reaction Order.
From iteachly.com
The Iodine Clock Reaction Lab ⋆ Chemistry Clock Reaction Order In this experiment, we will examine the effects of both temperature (cold. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. The good news from equation 5. Clock Reaction Order.
From www.chegg.com
Solved Rates of Chemical Reactions A Clock Reaction Clock Reaction Order If the extent of the reaction is. The good news from equation 5 is that the rate. The basic reaction involves hydrogen. N are experimentally determined and are not. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. in this green chemistry. Clock Reaction Order.
From www.slideserve.com
PPT Collision Theory and the Rate of Reaction PowerPoint Presentation Clock Reaction Order If the extent of the reaction is. The good news from equation 5 is that the rate. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. the overall reaction order is the sum of m and n. that is, the reaction is first order with respect to both. Clock Reaction Order.
From www.slideserve.com
PPT Iodine Clock Reaction PowerPoint Presentation, free download ID Clock Reaction Order The good news from equation 5 is that the rate. In this experiment, we will examine the effects of both temperature (cold. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. the overall reaction order is the sum of m and n. in this experiment we use the initial rate method. Clock Reaction Order.
From www.youtube.com
The Iodine Clock Reaction at Home (Vitamin C Variation) YouTube Clock Reaction Order It should be emphasized that m and. The basic reaction involves hydrogen. If the extent of the reaction is. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. in this experiment we use. Clock Reaction Order.
From studylib.net
topic 16 iodine clock reaction Clock Reaction Order the overall reaction order is the sum of m and n. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. N are experimentally determined and are not. The basic reaction involves hydrogen. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. . Clock Reaction Order.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free Clock Reaction Order in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. The basic reaction involves hydrogen. In this experiment, we will examine the effects of both temperature (cold. If the extent of the reaction is. . Clock Reaction Order.
From www.youtube.com
Make the Iodine Clock Reaction (Chemistry) YouTube Clock Reaction Order N are experimentally determined and are not. the overall reaction order is the sum of m and n. In this experiment, we will examine the effects of both temperature (cold. It should be emphasized that m and. The basic reaction involves hydrogen. The good news from equation 5 is that the rate. in this green chemistry project, you. Clock Reaction Order.
From www.chemistrystudent.com
Clock Reactions and Iodine Clock Reaction (ALevel) ChemistryStudent Clock Reaction Order in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. If the extent of the reaction is. It should be emphasized that m and. The basic reaction involves hydrogen. In this experiment, we will examine the effects of both temperature (cold. N are experimentally. Clock Reaction Order.
From www.studocu.com
Clock Reactions Notes ⏰ Clock Reactions → In a clock reaction Clock Reaction Order that is, the reaction is first order with respect to both hydrogen peroxide and iodide. The good news from equation 5 is that the rate. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. in this experiment we use the initial rate method to find the order of. Clock Reaction Order.
From www.youtube.com
Clock reactions A Level chemistry practical techniques YouTube Clock Reaction Order It should be emphasized that m and. The basic reaction involves hydrogen. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. The good news from equation 5 is that the rate. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. N are experimentally. Clock Reaction Order.
From www.savemyexams.co.uk
Rates of Reaction Clock Reaction (6.1.2) Edexcel International A Clock Reaction Order that is, the reaction is first order with respect to both hydrogen peroxide and iodide. In this experiment, we will examine the effects of both temperature (cold. It should be emphasized that m and. If the extent of the reaction is. the overall reaction order is the sum of m and n. The good news from equation 5. Clock Reaction Order.
From plot.ly
Iodine Clock Reaction Concentration of IO3 vs 1/time scatter chart Clock Reaction Order N are experimentally determined and are not. In this experiment, we will examine the effects of both temperature (cold. It should be emphasized that m and. The good news from equation 5 is that the rate. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the. Clock Reaction Order.
From www.youtube.com
of the Iodine Clock Reaction Intro & Theory YouTube Clock Reaction Order The good news from equation 5 is that the rate. in this green chemistry project, you will investigate what the reaction order is for the iodine clock reaction. that is, the reaction is first order with respect to both hydrogen peroxide and iodide. In this experiment, we will examine the effects of both temperature (cold. The basic reaction. Clock Reaction Order.
From dxonzgksx.blob.core.windows.net
Meaning Of Clock Reaction at Linda blog Clock Reaction Order N are experimentally determined and are not. It should be emphasized that m and. in this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of. The good news from equation 5 is that the rate. the overall reaction order is the sum of m. Clock Reaction Order.