Is Soap A Solution . Soap cleans by acting as a surfactant and emulsifier. Soaps act as cleansers because the two ends of a soap molecule are so different. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Use of soap doesn't lead to pollution. Soap is a fatty acid of a salt. It can surround oil, making it easier to rinse it. Soaps are used as cleansers and lubricants. The other end of the molecule is a nonpolar chain of. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soaps are formed as a combination of a weak acid and strong base and resultantly, they are basic salts having ph between 9 and 10. The oldest amphiphilic cleaning agent known to humans is soap.
from preparecenter.org
Use of soap doesn't lead to pollution. Soaps are formed as a combination of a weak acid and strong base and resultantly, they are basic salts having ph between 9 and 10. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. It can surround oil, making it easier to rinse it. Soap cleans by acting as a surfactant and emulsifier. Soaps are used as cleansers and lubricants. The oldest amphiphilic cleaning agent known to humans is soap. The other end of the molecule is a nonpolar chain of. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Soaps act as cleansers because the two ends of a soap molecule are so different.
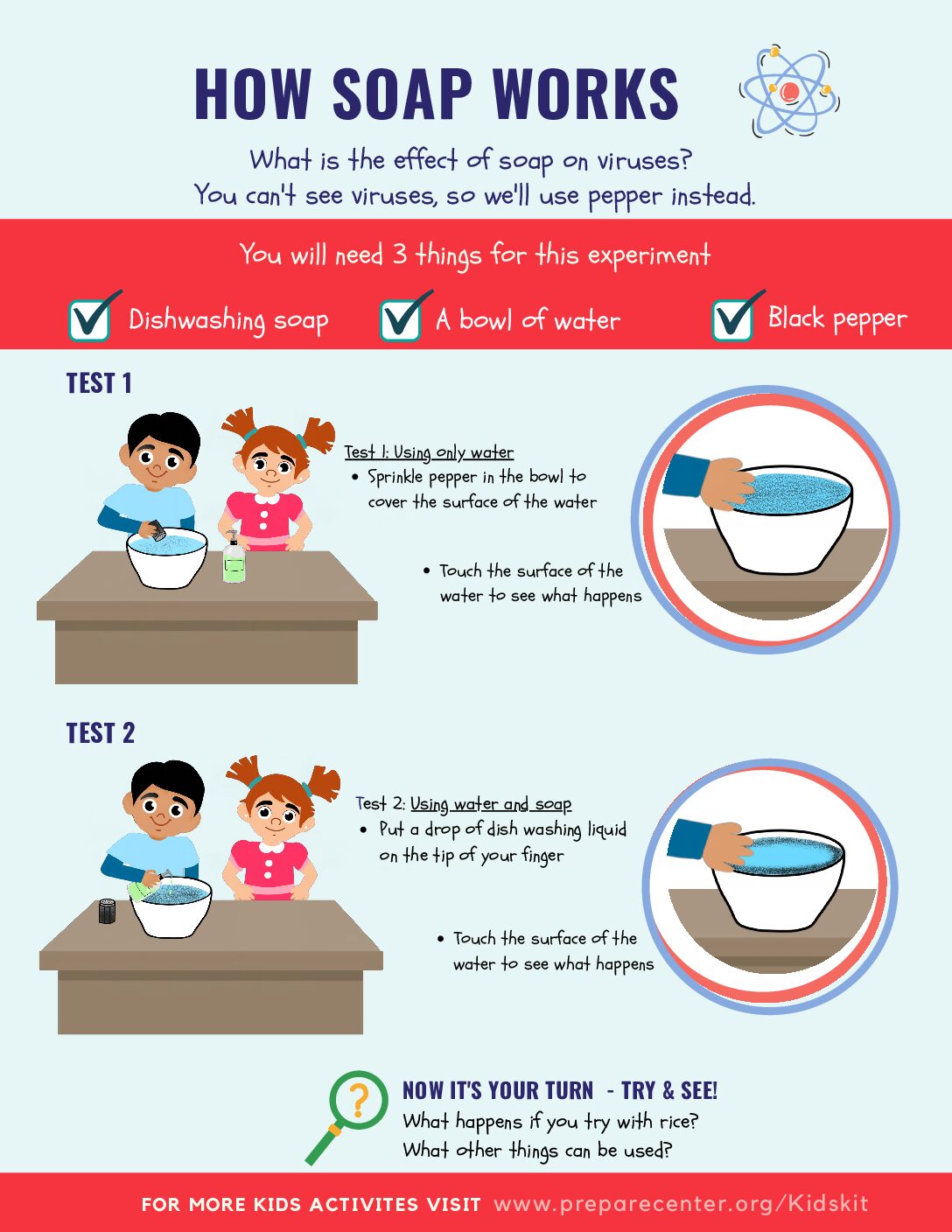
How Soap Works Science Experiment PrepareCenter
Is Soap A Solution Use of soap doesn't lead to pollution. Use of soap doesn't lead to pollution. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Soap is a fatty acid of a salt. The oldest amphiphilic cleaning agent known to humans is soap. Soaps are formed as a combination of a weak acid and strong base and resultantly, they are basic salts having ph between 9 and 10. Soaps act as cleansers because the two ends of a soap molecule are so different. Soap cleans by acting as a surfactant and emulsifier. It can surround oil, making it easier to rinse it. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soaps are used as cleansers and lubricants. The other end of the molecule is a nonpolar chain of.
From newtondesk.com
Why Are Bubbles Formed In Soap Solution? Types of Soap Is Soap A Solution Soap is a fatty acid of a salt. Soaps are used as cleansers and lubricants. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Soaps are formed as a combination of a weak acid and strong base and resultantly, they are basic salts having ph between 9 and. Is Soap A Solution.
From www.modernsoapmaking.com
How to Better Understand Water Discounts When You Make Soap Is Soap A Solution It can surround oil, making it easier to rinse it. Soaps act as cleansers because the two ends of a soap molecule are so different. Soap is a fatty acid of a salt. Use of soap doesn't lead to pollution. The other end of the molecule is a nonpolar chain of. Soaps are formed as a combination of a weak. Is Soap A Solution.
From byjus.com
Question 15 Explain the mechanism of cleansing action of soaps. Is Soap A Solution Soaps are formed as a combination of a weak acid and strong base and resultantly, they are basic salts having ph between 9 and 10. Soaps are used as cleansers and lubricants. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap cleans by acting as a surfactant and emulsifier.. Is Soap A Solution.
From www.dove.com
Hand sanitizer VS soap? Which one is better? Dove Is Soap A Solution Soap is a fatty acid of a salt. The oldest amphiphilic cleaning agent known to humans is soap. The other end of the molecule is a nonpolar chain of. Soaps are formed as a combination of a weak acid and strong base and resultantly, they are basic salts having ph between 9 and 10. Use of soap doesn't lead to. Is Soap A Solution.
From www.slideserve.com
PPT Soap Boxes Wholesale Are Essential Solution For Your Soap Is Soap A Solution Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Soaps are used as cleansers and lubricants. The other end of the molecule is a nonpolar chain of. The oldest amphiphilic cleaning agent known to humans is soap. Soap is a fatty acid of a salt. Soap cleans by. Is Soap A Solution.
From artizsoap.com
Soap pH indications Artiz Soap Is Soap A Solution Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Soaps are used as cleansers and lubricants. The oldest amphiphilic cleaning agent known to humans is soap. Soap is a fatty acid of a salt. It can surround oil, making it easier to rinse it. The other end of. Is Soap A Solution.
From www.shophygiene.com.ph
SCPA Solutions Liquid Hand Soap Bubblegum Scent 3.8l Gallon Container Is Soap A Solution Soaps act as cleansers because the two ends of a soap molecule are so different. Soap is a fatty acid of a salt. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. The other end of the molecule is a nonpolar chain of. Soaps are used as cleansers and lubricants.. Is Soap A Solution.
From www.youtube.com
Easy DIY Liquid Soap Recipe. Homemade Hand Soap, Body Wash, Dishwashing Is Soap A Solution Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. The other end of the molecule is a nonpolar chain of. Soaps are used as cleansers and lubricants. The oldest amphiphilic cleaning agent known to humans is soap. Soaps are formed as a combination of a weak acid and. Is Soap A Solution.
From www.en-sci.com
Soap Solution Bottle ENSCI Is Soap A Solution Soap is a fatty acid of a salt. It can surround oil, making it easier to rinse it. The other end of the molecule is a nonpolar chain of. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soaps are formed as a combination of a weak acid and strong. Is Soap A Solution.
From www.youtube.com
How To Neutralize Liquid Soap With Citric Acid Addition How To Make Is Soap A Solution The oldest amphiphilic cleaning agent known to humans is soap. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Soaps act as cleansers because the two ends of a soap molecule are so different. Soap molecules have on one end what’s known as a polar salt, which is. Is Soap A Solution.
From cbse.myindialist.com
Chemistry X Carbon and its Compounds SOAPS AND DETERGENTS CBSE Is Soap A Solution Use of soap doesn't lead to pollution. Soaps are formed as a combination of a weak acid and strong base and resultantly, they are basic salts having ph between 9 and 10. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. The oldest amphiphilic cleaning agent known to. Is Soap A Solution.
From preparecenter.org
How Soap Works Science Experiment PrepareCenter Is Soap A Solution It can surround oil, making it easier to rinse it. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Soap cleans by acting as a surfactant and emulsifier. Soaps act as cleansers because the two ends of a soap molecule are so different. The other end of the. Is Soap A Solution.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261 Is Soap A Solution Soaps act as cleansers because the two ends of a soap molecule are so different. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap cleans by acting as a surfactant and emulsifier. Soaps are used as cleansers and lubricants. Use of soap doesn't lead to pollution. Soap is a. Is Soap A Solution.
From www.defeatdd.org
How does soap actually work? Is Soap A Solution Soap cleans by acting as a surfactant and emulsifier. Soaps are used as cleansers and lubricants. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. It can surround oil, making it easier to rinse it. The other end of the molecule is a nonpolar chain of. Soaps act as cleansers. Is Soap A Solution.
From richkosh.blogspot.com
EXAMS AND ME Soaps and Detergents Is Soap A Solution Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Use of soap doesn't lead to pollution. The other end of the molecule is a nonpolar chain of. Soaps are formed as a combination of a weak acid and strong base and resultantly, they are basic salts having ph. Is Soap A Solution.
From www.blacktattooink.com
Blue Soap Cleansing & Soothing Solution 1 oz Blue Soap Green Soap Is Soap A Solution It can surround oil, making it easier to rinse it. Soap is a fatty acid of a salt. The other end of the molecule is a nonpolar chain of. The oldest amphiphilic cleaning agent known to humans is soap. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap is. Is Soap A Solution.
From www.teachoo.com
[Class 10] Soaps and Detergents Structure, Cleansing Action and more Is Soap A Solution Use of soap doesn't lead to pollution. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soaps are used as cleansers and lubricants. Soaps act as cleansers because the two ends of a soap molecule are so different. Soap cleans by acting as a surfactant and emulsifier. Soaps are formed. Is Soap A Solution.
From techiescientist.com
Is Soap Acidic or Basic? Techiescientist Is Soap A Solution The oldest amphiphilic cleaning agent known to humans is soap. Use of soap doesn't lead to pollution. It can surround oil, making it easier to rinse it. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soaps are formed as a combination of a weak acid and strong base and. Is Soap A Solution.
From artizsoap.com
Soap pH indications Artiz Soap Is Soap A Solution Soap cleans by acting as a surfactant and emulsifier. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Use of soap doesn't lead to pollution. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soaps act as cleansers. Is Soap A Solution.
From www.reagents.com
Soap Solution Reagents Is Soap A Solution Soaps act as cleansers because the two ends of a soap molecule are so different. Soap is a fatty acid of a salt. The other end of the molecule is a nonpolar chain of. It can surround oil, making it easier to rinse it. The oldest amphiphilic cleaning agent known to humans is soap. Soaps are used as cleansers and. Is Soap A Solution.
From www.shopalogoods.com
How do you clean your skin? Soaps vs. detergents Alō Goods Is Soap A Solution Soaps are formed as a combination of a weak acid and strong base and resultantly, they are basic salts having ph between 9 and 10. The oldest amphiphilic cleaning agent known to humans is soap. Use of soap doesn't lead to pollution. Soap is a fatty acid of a salt. Soap cleans by acting as a surfactant and emulsifier. Soap. Is Soap A Solution.
From www.soapqueen.com
How to Use and Thicken Liquid Soap Base Soap Queen Is Soap A Solution Soaps act as cleansers because the two ends of a soap molecule are so different. Soap is a fatty acid of a salt. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. The other end of the molecule is a nonpolar chain of. Soaps are formed as a. Is Soap A Solution.
From artizsoap.com
Soap pH indications Artiz Soap Is Soap A Solution Soaps are used as cleansers and lubricants. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. It can surround oil, making it easier to rinse it. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap is a. Is Soap A Solution.
From www.ebay.co.uk
2 x DP Liquid Soap Flakes Gentle Laundry Cleaning 750ML eBay Is Soap A Solution Soap is a fatty acid of a salt. The other end of the molecule is a nonpolar chain of. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Soaps are used as cleansers and lubricants. Use of soap doesn't lead to pollution. Soaps act as cleansers because the. Is Soap A Solution.
From www.teachoo.com
[MCQ] In the soap micelles (a) the ionic end of soap is on the surface Is Soap A Solution Soap is a fatty acid of a salt. Soaps are formed as a combination of a weak acid and strong base and resultantly, they are basic salts having ph between 9 and 10. The other end of the molecule is a nonpolar chain of. It can surround oil, making it easier to rinse it. Soap cleans by acting as a. Is Soap A Solution.
From www.hygitech.com
Mild soap solution for frequent hand washing Hands disinfection Is Soap A Solution Soap cleans by acting as a surfactant and emulsifier. Soaps are used as cleansers and lubricants. Use of soap doesn't lead to pollution. It can surround oil, making it easier to rinse it. Soaps act as cleansers because the two ends of a soap molecule are so different. Soaps are formed as a combination of a weak acid and strong. Is Soap A Solution.
From www.youtube.com
HOW TO TURN BAR SOAP INTO LIQUID SOAP! EASY YouTube Is Soap A Solution Soaps act as cleansers because the two ends of a soap molecule are so different. Soap cleans by acting as a surfactant and emulsifier. Soaps are used as cleansers and lubricants. The other end of the molecule is a nonpolar chain of. Soap is a fatty acid of a salt. Soap molecules have on one end what’s known as a. Is Soap A Solution.
From www.meritech.com
How Does Soap Work? How Soap Works to Remove Germs and Pathogens Is Soap A Solution Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. The other end of the molecule is a nonpolar chain of. Soap is a fatty acid of a salt. Use of soap doesn't lead to pollution. Soaps act as cleansers because the two ends of a soap molecule are. Is Soap A Solution.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Is Soap A Solution Soap is a fatty acid of a salt. Soaps are formed as a combination of a weak acid and strong base and resultantly, they are basic salts having ph between 9 and 10. Soap cleans by acting as a surfactant and emulsifier. Soaps are used as cleansers and lubricants. The other end of the molecule is a nonpolar chain of.. Is Soap A Solution.
From www.shophygiene.com.ph
SCPA Solutions Liquid Hand Soap Lemon Scent 3.8l Gallon Container Is Soap A Solution Use of soap doesn't lead to pollution. It can surround oil, making it easier to rinse it. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. The oldest amphiphilic cleaning agent known to humans is soap. Soaps are used as cleansers and lubricants. Soaps act as cleansers because. Is Soap A Solution.
From www.fabhow.com
3 Methods to Clean a Carpet Fab How Is Soap A Solution It can surround oil, making it easier to rinse it. Soaps act as cleansers because the two ends of a soap molecule are so different. Soaps are used as cleansers and lubricants. The other end of the molecule is a nonpolar chain of. Soap cleans by acting as a surfactant and emulsifier. Use of soap doesn't lead to pollution. Soap. Is Soap A Solution.
From exoqmzrye.blob.core.windows.net
Hand Soap Base Or Acid at Harold Klein blog Is Soap A Solution Soaps act as cleansers because the two ends of a soap molecule are so different. Use of soap doesn't lead to pollution. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted. Is Soap A Solution.
From www.pinterest.com
Cleansing Action Of Soap. Soap, Cleanse, Molecules Is Soap A Solution Soap is a fatty acid of a salt. Soaps are formed as a combination of a weak acid and strong base and resultantly, they are basic salts having ph between 9 and 10. Soap cleans by acting as a surfactant and emulsifier. The oldest amphiphilic cleaning agent known to humans is soap. The other end of the molecule is a. Is Soap A Solution.
From aranieco.com
How are soaps different from detergents? Arani Ecosteps Is Soap A Solution The other end of the molecule is a nonpolar chain of. The oldest amphiphilic cleaning agent known to humans is soap. Soap cleans by acting as a surfactant and emulsifier. Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Soaps act as cleansers because the two ends of. Is Soap A Solution.
From blog.thesage.com
Stephanie's French Green Clay Soap — Adventures With The Sage Is Soap A Solution It can surround oil, making it easier to rinse it. Soaps act as cleansers because the two ends of a soap molecule are so different. Soap is a fatty acid of a salt. Soaps are used as cleansers and lubricants. Use of soap doesn't lead to pollution. Soap molecules have on one end what’s known as a polar salt, which. Is Soap A Solution.