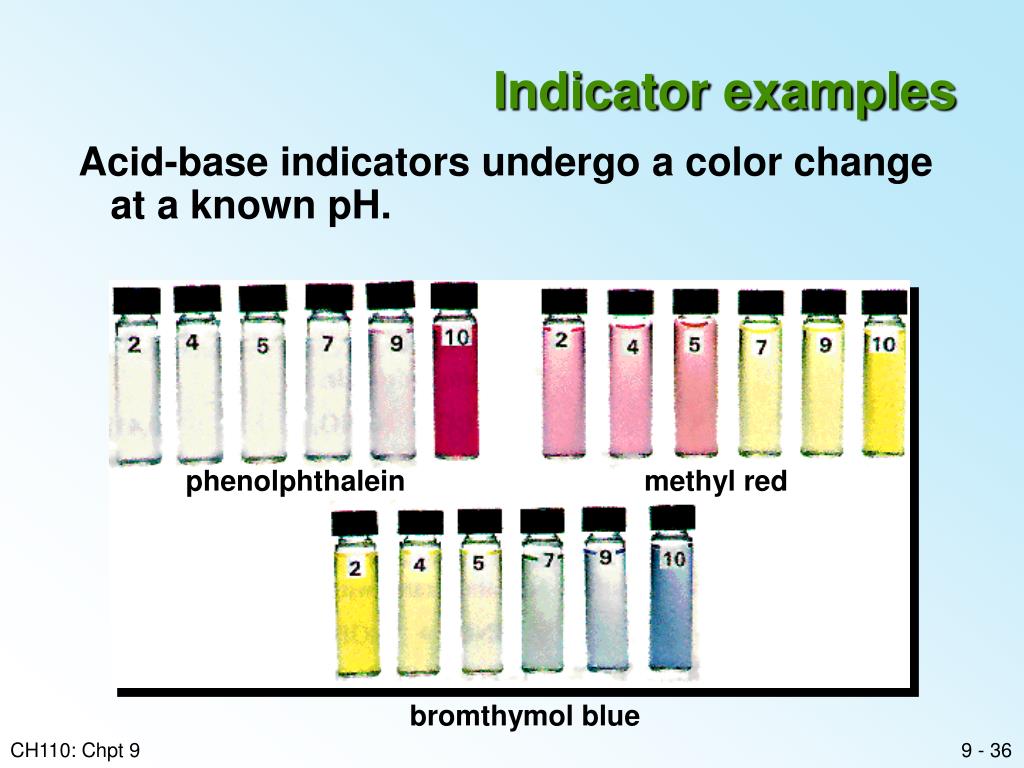
Purpose Of Indicator Acid Base . When titrating a strong acid or base, the endpoint should occur at a ph of 7. The undissociated form of the indicator is a different color than the iogenic form of the indicator. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Only a small amount of indicator compound is needed to produce a visible color change. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. They are chosen based on the desired endpoint and the. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are typically weak acids or bases whose changes in color.
from www.slideserve.com
Acid and base indicators are usually used to detect the endpoint of acid and base titrations. Only a small amount of indicator compound is needed to produce a visible color change. When titrating a strong acid or base, the endpoint should occur at a ph of 7. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. The undissociated form of the indicator is a different color than the iogenic form of the indicator. They are chosen based on the desired endpoint and the. They are typically weak acids or bases whose changes in color. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units.
PPT Acids and Bases PowerPoint Presentation, free download ID5741622
Purpose Of Indicator Acid Base We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. The undissociated form of the indicator is a different color than the iogenic form of the indicator. Only a small amount of indicator compound is needed to produce a visible color change. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. They are chosen based on the desired endpoint and the. They are typically weak acids or bases whose changes in color. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. When titrating a strong acid or base, the endpoint should occur at a ph of 7.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5123561 Purpose Of Indicator Acid Base The undissociated form of the indicator is a different color than the iogenic form of the indicator. Only a small amount of indicator compound is needed to produce a visible color change. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are chosen based on the desired endpoint and the.. Purpose Of Indicator Acid Base.
From slideplayer.com
Indicators. ppt download Purpose Of Indicator Acid Base To be effective, the right indicator must be used that changes color as close to the endpoint as possible. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. When titrating a strong acid or base, the endpoint should occur at a ph of 7. They are chosen based on the desired. Purpose Of Indicator Acid Base.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Purpose Of Indicator Acid Base To be effective, the right indicator must be used that changes color as close to the endpoint as possible. Only a small amount of indicator compound is needed to produce a visible color change. When titrating a strong acid or base, the endpoint should occur at a ph of 7. Acid and base indicators are usually used to detect the. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT Acid, Base, Universal Indicator and PH Scale PowerPoint Purpose Of Indicator Acid Base Only a small amount of indicator compound is needed to produce a visible color change. They are chosen based on the desired endpoint and the. When titrating a strong acid or base, the endpoint should occur at a ph of 7. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. We can represent. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID Purpose Of Indicator Acid Base They are typically weak acids or bases whose changes in color. Only a small amount of indicator compound is needed to produce a visible color change. When titrating a strong acid or base, the endpoint should occur at a ph of 7. They are chosen based on the desired endpoint and the. The undissociated form of the indicator is a. Purpose Of Indicator Acid Base.
From www.studypool.com
SOLUTION Theory of acid base indicators with detailed notes Studypool Purpose Of Indicator Acid Base Acid and base indicators are usually used to detect the endpoint of acid and base titrations. They are typically weak acids or bases whose changes in color. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. The undissociated form of the indicator is a different color than the iogenic form of. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID546616 Purpose Of Indicator Acid Base We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are chosen based on the desired endpoint and the. When titrating a strong acid or base, the endpoint should occur at a ph of 7. The undissociated form of the indicator is a different color than the iogenic form of the. Purpose Of Indicator Acid Base.
From slideplayer.com
PART 1 Acid/Base Theory & Properties ppt download Purpose Of Indicator Acid Base When titrating a strong acid or base, the endpoint should occur at a ph of 7. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. They are chosen based on the desired. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT 6.3 AcidBase Indicators PowerPoint Presentation, free download Purpose Of Indicator Acid Base The undissociated form of the indicator is a different color than the iogenic form of the indicator. When titrating a strong acid or base, the endpoint should occur at a ph of 7. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are typically weak acids or bases whose changes. Purpose Of Indicator Acid Base.
From www.scribd.com
ACIDBASE INDICATORS 211.ppt Acid Ph Purpose Of Indicator Acid Base Only a small amount of indicator compound is needed to produce a visible color change. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are chosen based on the desired endpoint and the. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. When. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2089759 Purpose Of Indicator Acid Base An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. Only a small amount of indicator compound is needed to produce a visible color change. They are chosen based on the desired endpoint. Purpose Of Indicator Acid Base.
From www.youtube.com
Acid Base Indicators Introduction Acids and Bases Chemistry Practice Purpose Of Indicator Acid Base The undissociated form of the indicator is a different color than the iogenic form of the indicator. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. They are typically weak acids or bases whose changes in color. To be effective, the right indicator must be used that changes color as close to the. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation, free download ID3840510 Purpose Of Indicator Acid Base The undissociated form of the indicator is a different color than the iogenic form of the indicator. When titrating a strong acid or base, the endpoint should occur at a ph of 7. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. They are typically weak acids or bases whose changes in color.. Purpose Of Indicator Acid Base.
From exocsufck.blob.core.windows.net
Ph Indicator Acid Base at Maria Usher blog Purpose Of Indicator Acid Base They are typically weak acids or bases whose changes in color. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. The undissociated form of the indicator is a different color than the iogenic form. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT ACIDBASE INDICATORS PowerPoint Presentation, free download ID Purpose Of Indicator Acid Base To be effective, the right indicator must be used that changes color as close to the endpoint as possible. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Only a small amount of indicator compound is needed to produce a visible color change. The undissociated form of the indicator is a. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation, free download ID3840510 Purpose Of Indicator Acid Base They are chosen based on the desired endpoint and the. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. The undissociated form of the indicator is a different color than the iogenic form of the indicator. They are typically weak acids or bases whose changes in color. Acid and base indicators. Purpose Of Indicator Acid Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Purpose Of Indicator Acid Base To be effective, the right indicator must be used that changes color as close to the endpoint as possible. Only a small amount of indicator compound is needed to produce a visible color change. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are chosen based on the desired endpoint. Purpose Of Indicator Acid Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Purpose Of Indicator Acid Base They are chosen based on the desired endpoint and the. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. They are typically weak acids or bases whose changes in color. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Acid and. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT PROPERTIES OF ACIDS & BASES PowerPoint Presentation, free Purpose Of Indicator Acid Base The undissociated form of the indicator is a different color than the iogenic form of the indicator. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. An indicator is a weak acid that ionizes. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free Purpose Of Indicator Acid Base They are typically weak acids or bases whose changes in color. Only a small amount of indicator compound is needed to produce a visible color change. When titrating a strong acid or base, the endpoint should occur at a ph of 7. To be effective, the right indicator must be used that changes color as close to the endpoint as. Purpose Of Indicator Acid Base.
From slideplayer.com
Experiment 5 Statistical Evaluation of AcidBase Indicators ppt download Purpose Of Indicator Acid Base The undissociated form of the indicator is a different color than the iogenic form of the indicator. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. Acid and base indicators are usually. Purpose Of Indicator Acid Base.
From pediaa.com
Difference Between Acid Base Indicator and Universal Indicator Purpose Of Indicator Acid Base Only a small amount of indicator compound is needed to produce a visible color change. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. When titrating a strong acid or base, the endpoint. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation ID4827855 Purpose Of Indicator Acid Base An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are typically weak acids. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT Acid/Base Indicators PowerPoint Presentation, free download ID Purpose Of Indicator Acid Base Only a small amount of indicator compound is needed to produce a visible color change. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. They are chosen based on the desired endpoint and the. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. An. Purpose Of Indicator Acid Base.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Purpose Of Indicator Acid Base Acid and base indicators are usually used to detect the endpoint of acid and base titrations. The undissociated form of the indicator is a different color than the iogenic form of the indicator. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are typically weak acids or bases whose changes. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT Acids, Bases, and AcidBase Equilibria PowerPoint Presentation Purpose Of Indicator Acid Base To be effective, the right indicator must be used that changes color as close to the endpoint as possible. They are chosen based on the desired endpoint and the. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. An indicator is a weak acid that ionizes within a known ph range,. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT AcidBase Equilibrium PowerPoint Presentation, free download ID Purpose Of Indicator Acid Base When titrating a strong acid or base, the endpoint should occur at a ph of 7. They are typically weak acids or bases whose changes in color. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Purpose Of Indicator Acid Base.
From foundoutaboutchemistry.blogspot.com
Found Out About Chemistry Acidbase indicator charts Purpose Of Indicator Acid Base An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. The undissociated form of the indicator is a different color than the iogenic form of the indicator. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. They are chosen based on the desired endpoint and. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT ACID BASE REACTIONS PowerPoint Presentation, free download ID Purpose Of Indicator Acid Base Only a small amount of indicator compound is needed to produce a visible color change. When titrating a strong acid or base, the endpoint should occur at a ph of 7. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are chosen based on the desired endpoint and the. They. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5741622 Purpose Of Indicator Acid Base They are typically weak acids or bases whose changes in color. The undissociated form of the indicator is a different color than the iogenic form of the indicator. When titrating a strong acid or base, the endpoint should occur at a ph of 7. Acid and base indicators are usually used to detect the endpoint of acid and base titrations.. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT Acid/Base Indicators PowerPoint Presentation, free download ID Purpose Of Indicator Acid Base Only a small amount of indicator compound is needed to produce a visible color change. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. When titrating a strong acid or base, the endpoint should occur at a ph of 7. The undissociated form of the indicator is a different color than. Purpose Of Indicator Acid Base.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Purpose Of Indicator Acid Base They are typically weak acids or bases whose changes in color. The undissociated form of the indicator is a different color than the iogenic form of the indicator. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are chosen based on the desired endpoint and the. Acid and base indicators. Purpose Of Indicator Acid Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Purpose Of Indicator Acid Base The undissociated form of the indicator is a different color than the iogenic form of the indicator. They are chosen based on the desired endpoint and the. When titrating a strong acid or base, the endpoint should occur at a ph of 7. They are typically weak acids or bases whose changes in color. We can represent the protonated form. Purpose Of Indicator Acid Base.
From www.slideserve.com
PPT AcidBase Indicators PowerPoint Presentation, free download ID Purpose Of Indicator Acid Base An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. The undissociated form of the indicator is a different color than the iogenic form of the indicator. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. When titrating a strong acid or. Purpose Of Indicator Acid Base.
From www.labkafe.com
Universal Indicator ‒ What is it, Why Do We Need It, and How to Use Purpose Of Indicator Acid Base They are chosen based on the desired endpoint and the. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. Only a small amount of indicator compound is needed to produce a visible color change. When. Purpose Of Indicator Acid Base.