Is Charcoal Burning A Redox Reaction . organic chemists use a variety of redox reactions. Taking the place of oxygen. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. making charcoal burn faster: Hsiao shih, saltpeter, potassium nitrate. The theory was also applied to processes other than. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough.
from slideplayer.com
in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. Hsiao shih, saltpeter, potassium nitrate. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. organic chemists use a variety of redox reactions. making charcoal burn faster: Taking the place of oxygen. The theory was also applied to processes other than. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough.
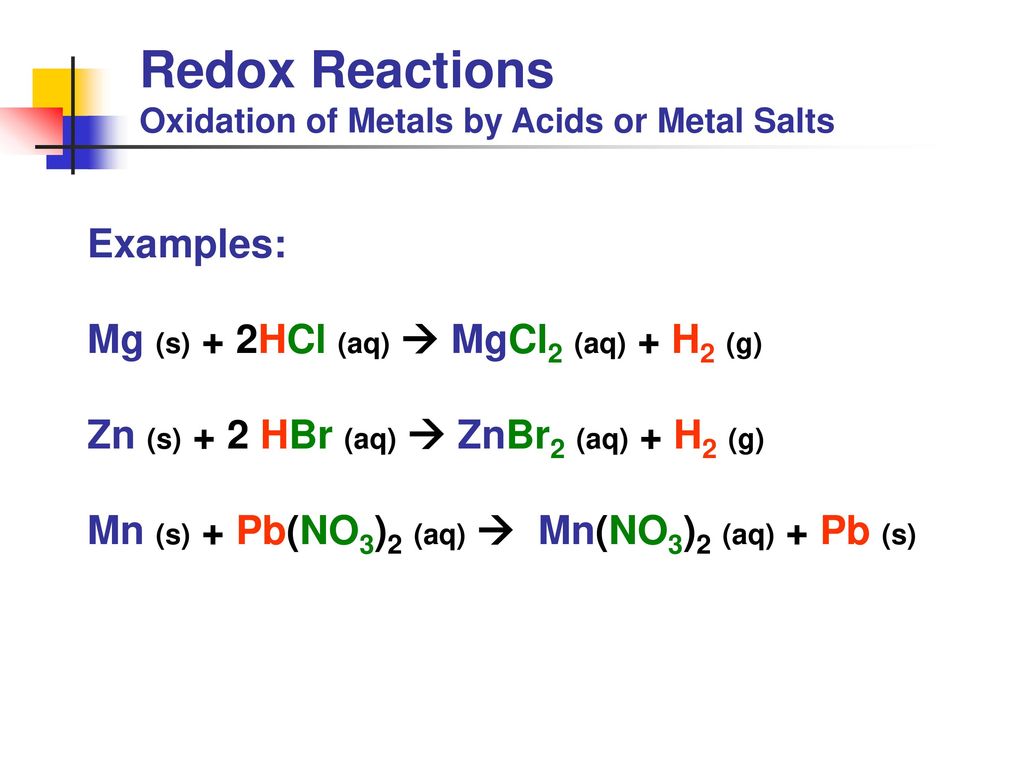
Redox Reactions Many practical or everyday examples of redox reactions
Is Charcoal Burning A Redox Reaction making charcoal burn faster: thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. Hsiao shih, saltpeter, potassium nitrate. The theory was also applied to processes other than. making charcoal burn faster: Taking the place of oxygen. charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. organic chemists use a variety of redox reactions. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is.
From www.slideserve.com
PPT Ch. 20 OxidationReduction Reactions PowerPoint Presentation Is Charcoal Burning A Redox Reaction The theory was also applied to processes other than. making charcoal burn faster: in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. Taking the place. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Redox Reactions Many practical or everyday examples of redox reactions Is Charcoal Burning A Redox Reaction For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. Taking the place of oxygen. making charcoal burn faster: charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough.. Is Charcoal Burning A Redox Reaction.
From owlcation.com
AS Chemistry Redox Reactions and Group 2 Elements Owlcation Is Charcoal Burning A Redox Reaction in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. making charcoal burn faster: Taking the place of oxygen. charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. thus, the burning of charcoal. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Redox Reactions Many practical or everyday examples of redox reactions Is Charcoal Burning A Redox Reaction Taking the place of oxygen. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. The theory was also applied to processes other than. organic chemists use a variety of redox reactions. Hsiao shih, saltpeter, potassium nitrate. charcoal isn't passivating, just the last component to burn and,. Is Charcoal Burning A Redox Reaction.
From dokumen.tips
(PPT) OxidationReduction Reactions (OxRedox) examples rusting Is Charcoal Burning A Redox Reaction charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. Hsiao shih, saltpeter, potassium nitrate. making charcoal burn faster: thus, the burning of charcoal was. Is Charcoal Burning A Redox Reaction.
From www.aakash.ac.in
Redox Reactions Definition, Types, Applications & Uses Chemistry Is Charcoal Burning A Redox Reaction For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. Taking the place of oxygen. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. The theory was also. Is Charcoal Burning A Redox Reaction.
From peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Is Charcoal Burning A Redox Reaction For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. The theory was also applied to processes other than. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. Taking. Is Charcoal Burning A Redox Reaction.
From pendulumedu.com
Oxidation and Reduction Redox Reactions, Definitions, Examples Is Charcoal Burning A Redox Reaction in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. Taking the place of oxygen. The theory was also applied to processes other than. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. making charcoal. Is Charcoal Burning A Redox Reaction.
From stock.adobe.com
illustration of physics and chemistry, Combustion, Burning is a high Is Charcoal Burning A Redox Reaction For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. Hsiao shih, saltpeter, potassium nitrate. The theory was also applied to processes other than. organic chemists use a variety of redox reactions. in a combustion reaction, the thing that burns (the reactant that. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Redox Reactions Many practical or everyday examples of redox reactions Is Charcoal Burning A Redox Reaction The theory was also applied to processes other than. Hsiao shih, saltpeter, potassium nitrate. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Redox Reactions Many practical or everyday examples of redox reactions Is Charcoal Burning A Redox Reaction charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. organic chemists use a variety of redox reactions. making charcoal burn faster: in a combustion reaction, the. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Chapter 4 Type of Chemical Reactions and Solution Stoichiometric ppt Is Charcoal Burning A Redox Reaction organic chemists use a variety of redox reactions. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. Taking the place of oxygen. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the. Is Charcoal Burning A Redox Reaction.
From slidetodoc.com
7 1 Describing Reactions Burning is a chemical Is Charcoal Burning A Redox Reaction in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. making charcoal burn faster: organic chemists use a variety of redox reactions. Hsiao shih, saltpeter, potassium nitrate. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. in a combustion reaction, the thing that burns (the reactant that isn't. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Chapter 20 OxidationReduction Reactions (Redox Reactions) ppt download Is Charcoal Burning A Redox Reaction making charcoal burn faster: The theory was also applied to processes other than. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. Taking the place. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Redox Reactions Many practical or everyday examples of redox reactions Is Charcoal Burning A Redox Reaction making charcoal burn faster: The theory was also applied to processes other than. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. organic chemists use a variety of redox reactions. charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. Hsiao shih, saltpeter, potassium nitrate. in the. Is Charcoal Burning A Redox Reaction.
From www.slideserve.com
PPT Chapter 19 Oxidation Reduction Reactions PowerPoint Is Charcoal Burning A Redox Reaction making charcoal burn faster: in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. Hsiao shih, saltpeter, potassium nitrate. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. organic chemists use. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Redox Reactions Many practical or everyday examples of redox reactions Is Charcoal Burning A Redox Reaction charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. Taking the place of oxygen. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. Hsiao shih, saltpeter, potassium nitrate. The theory was also applied to processes other than. in the traditional version of this pyrolysis process, called charcoal burning,. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Bonding, Replacements, Redox ppt download Is Charcoal Burning A Redox Reaction For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. The theory was also applied to processes other than. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. Hsiao shih, saltpeter, potassium nitrate. organic chemists use a variety of redox reactions. thus, the burning of charcoal was interpreted as. Is Charcoal Burning A Redox Reaction.
From sciencenotes.org
Redox Reactions Identify and Balance Oxidation and Reduction Is Charcoal Burning A Redox Reaction organic chemists use a variety of redox reactions. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. The theory was also applied to processes other than. Hsiao shih, saltpeter,. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Redox Reactions Many practical or everyday examples of redox reactions Is Charcoal Burning A Redox Reaction thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. The theory was also applied to processes other than. organic chemists use a variety of redox reactions. making charcoal burn faster: in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is. Is Charcoal Burning A Redox Reaction.
From dokumen.tips
(PPTX) Chemical Reactions Burning is an example of a chemical reaction Is Charcoal Burning A Redox Reaction organic chemists use a variety of redox reactions. Hsiao shih, saltpeter, potassium nitrate. making charcoal burn faster: in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called. Is Charcoal Burning A Redox Reaction.
From slidetodoc.com
Types of Chemical Reactions Types of Chemical Reactions Is Charcoal Burning A Redox Reaction making charcoal burn faster: organic chemists use a variety of redox reactions. Hsiao shih, saltpeter, potassium nitrate. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. The theory was also applied to processes other than. charcoal isn't. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Redox Reactions Many practical or everyday examples of redox reactions Is Charcoal Burning A Redox Reaction in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. The theory was also applied to processes other than. Taking. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Redox Reactions Many practical or everyday examples of redox reactions Is Charcoal Burning A Redox Reaction in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. The theory was also applied to processes other than. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. organic chemists use a variety of redox. Is Charcoal Burning A Redox Reaction.
From www.numerade.com
SOLVED Which of the following are redox reactions? (Select all of the Is Charcoal Burning A Redox Reaction The theory was also applied to processes other than. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. Taking the place of oxygen. making charcoal burn faster: thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. For example,. Is Charcoal Burning A Redox Reaction.
From scienceinfo.com
Redox Reactions 4 Types, Significance, Examples Is Charcoal Burning A Redox Reaction thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. organic chemists use a variety of redox reactions. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. making charcoal burn faster: Hsiao shih, saltpeter, potassium nitrate. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or. Is Charcoal Burning A Redox Reaction.
From www.slideserve.com
PPT Unit 5 Part 2 Redox Reactions and Electrochemistry PowerPoint Is Charcoal Burning A Redox Reaction organic chemists use a variety of redox reactions. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. making charcoal burn faster: For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Redox Reactions Many practical or everyday examples of redox reactions Is Charcoal Burning A Redox Reaction thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. making charcoal burn faster: charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. in a combustion reaction, the thing that burns (the reactant that isn't o 2. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Redox Reactions Many practical or everyday examples of redox reactions Is Charcoal Burning A Redox Reaction For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. Hsiao shih, saltpeter, potassium nitrate. making charcoal burn faster: The theory was also applied to processes other than. in the traditional version of this pyrolysis process, called charcoal burning,. Is Charcoal Burning A Redox Reaction.
From slideplayer.com
Redox Reactions Many practical or everyday examples of redox reactions Is Charcoal Burning A Redox Reaction in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. Hsiao shih, saltpeter, potassium nitrate. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. in the traditional. Is Charcoal Burning A Redox Reaction.
From eduinput.com
10 Examples of Redox Reactions Is Charcoal Burning A Redox Reaction in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. For example, potassium dichromate (\(\ce{k2cr2o7}\)) is a. organic chemists use a variety of redox reactions. Taking the place of oxygen.. Is Charcoal Burning A Redox Reaction.
From www.worksheetsplanet.com
What is a REDOX Reaction? Is Charcoal Burning A Redox Reaction Hsiao shih, saltpeter, potassium nitrate. organic chemists use a variety of redox reactions. The theory was also applied to processes other than. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the fuel. making charcoal burn faster: charcoal isn't passivating, just the last component to burn. Is Charcoal Burning A Redox Reaction.
From giogaaphs.blob.core.windows.net
Wood Burning Chemical Reaction at Kyle Parra blog Is Charcoal Burning A Redox Reaction in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. charcoal isn't passivating, just the last component to burn and, if the fire isn't well constructed, there may never be enough. thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the. Is Charcoal Burning A Redox Reaction.
From sciencenotes.org
Redox Reactions Identify and Balance Oxidation and Reduction Is Charcoal Burning A Redox Reaction thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. in a combustion reaction, the thing that burns (the reactant that isn't o 2 or f 2) is called the. Is Charcoal Burning A Redox Reaction.
From edenmeowbeard.blogspot.com
Redox Reaction Meaning Is Charcoal Burning A Redox Reaction Hsiao shih, saltpeter, potassium nitrate. thus, the burning of charcoal was interpreted as the loss of phlogiston from carbon to the air. organic chemists use a variety of redox reactions. in the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is. The theory was also applied to processes. Is Charcoal Burning A Redox Reaction.