Titration And Calculations . And are the volumes of the acid and base, respectively. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. The second example addresses a weak acid titration requiring equilibrium calculations. Moles of acid = 0.05 x 25/1000. Titration calculations generally involve this equation: Is the molarity of the acid, while is the molarity of the base. Moles of acid = concentration x volume in dm 3. You want to measure the volumes at the. Find the number of moles of acid. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Calculate the concentration of the sodium hydroxide solution. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.
from www.chegg.com
The second example addresses a weak acid titration requiring equilibrium calculations. You want to measure the volumes at the. And are the volumes of the acid and base, respectively. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. Titration calculations generally involve this equation: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Is the molarity of the acid, while is the molarity of the base. Moles of acid = 0.05 x 25/1000. Moles of acid = concentration x volume in dm 3. Calculate the concentration of the sodium hydroxide solution.
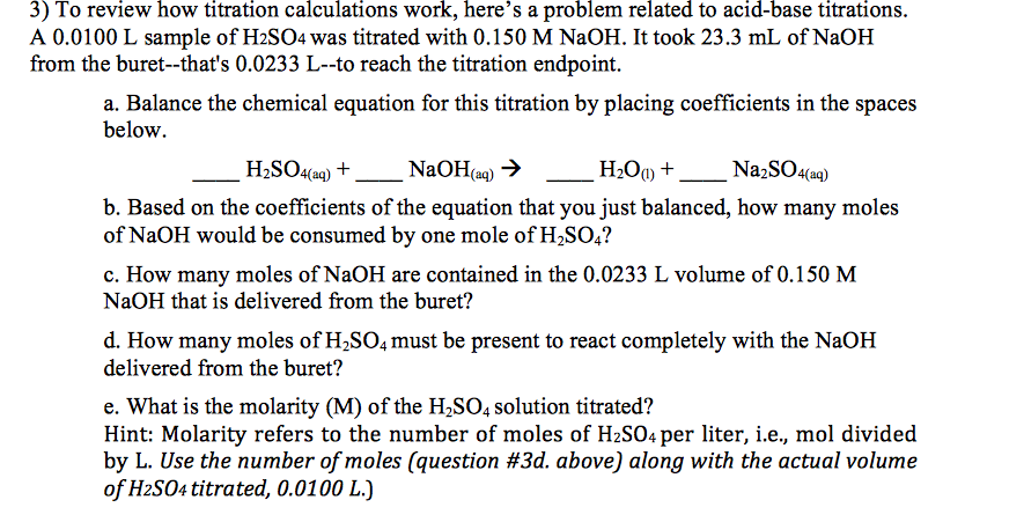
Solved To review how titration calculations work, here's a
Titration And Calculations A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Find the number of moles of acid. The second example addresses a weak acid titration requiring equilibrium calculations. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Moles of acid = concentration x volume in dm 3. Calculate the concentration of the sodium hydroxide solution. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Moles of acid = 0.05 x 25/1000. You want to measure the volumes at the. And are the volumes of the acid and base, respectively. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration calculations generally involve this equation: Is the molarity of the acid, while is the molarity of the base.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration And Calculations Find the number of moles of acid. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Is the molarity of the acid, while is the molarity of the base. You want to measure the volumes at the. And are the volumes of the acid and base, respectively. Titration calculations. Titration And Calculations.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration And Calculations You want to measure the volumes at the. Moles of acid = 0.05 x 25/1000. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Find the number of moles of acid. Titration calculations generally involve this equation: Calculate the concentration of the sodium hydroxide solution. A titration curve is. Titration And Calculations.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration And Calculations Titration calculations generally involve this equation: And are the volumes of the acid and base, respectively. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. The second example addresses a weak acid titration requiring equilibrium calculations. Find the number of. Titration And Calculations.
From www.tes.com
Measurement calculations/uncertainty for Titrations AS Chemistry Titration And Calculations You want to measure the volumes at the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The second example addresses a weak acid titration requiring equilibrium calculations. Is the molarity of the acid, while is the molarity of the base. A titration is a volumetric technique in which a solution. Titration And Calculations.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration And Calculations Calculate the concentration of the sodium hydroxide solution. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence.. Titration And Calculations.
From www.studocu.com
Chapter 14 Acid Base Titration Calculations in the context of Titration And Calculations The second example addresses a weak acid titration requiring equilibrium calculations. Find the number of moles of acid. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Calculate the concentration of the sodium hydroxide solution. A titration curve is a plot of the concentration of the analyte at a given point. Titration And Calculations.
From morioh.com
Acid Base Titration Curves PH Calculations Titration And Calculations And are the volumes of the acid and base, respectively. The second example addresses a weak acid titration requiring equilibrium calculations. Titration calculations generally involve this equation: Is the molarity of the acid, while is the molarity of the base. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Calculate the. Titration And Calculations.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration And Calculations A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Is the molarity of the acid, while is the molarity of the base. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.. Titration And Calculations.
From www.science-revision.co.uk
Titrations Titration And Calculations A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Titration calculations generally involve this equation: The second example addresses a weak acid titration requiring equilibrium calculations. Find the number of moles of acid. Moles of acid = 0.05 x 25/1000.. Titration And Calculations.
From www.chegg.com
Solved To review how titration calculations work, here's a Titration And Calculations Moles of acid = concentration x volume in dm 3. The second example addresses a weak acid titration requiring equilibrium calculations. Titration calculations generally involve this equation: A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. A titration is a volumetric technique in which a solution. Titration And Calculations.
From bazaemmalyman.blogspot.com
Back Titration Method Emma Lyman Titration And Calculations The second example addresses a weak acid titration requiring equilibrium calculations. Find the number of moles of acid. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Calculate the concentration of the sodium hydroxide solution. Moles of acid = 0.05 x 25/1000. And are the volumes of the acid. Titration And Calculations.
From chem4three.blogspot.ca
CHEMISTRY 11 TITRATIONS Titration And Calculations A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Is the molarity of the acid, while is the molarity of the base. Calculate the concentration of the sodium. Titration And Calculations.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Titration And Calculations And are the volumes of the acid and base, respectively. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Moles of acid = 0.05 x 25/1000. Find the number of moles of acid. A titration is a laboratory technique used. Titration And Calculations.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration And Calculations Is the molarity of the acid, while is the molarity of the base. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. And are the volumes of the acid and base, respectively. A titration curve is a plot of the concentration of the analyte at a given point in the experiment. Titration And Calculations.
From www.vrogue.co
7 Inspiration Titration Calculations Worksheet Gcse M vrogue.co Titration And Calculations And are the volumes of the acid and base, respectively. Is the molarity of the acid, while is the molarity of the base. The second example addresses a weak acid titration requiring equilibrium calculations. Find the number of moles of acid. Titration calculations generally involve this equation: Calculate the concentration of the sodium hydroxide solution. You want to measure the. Titration And Calculations.
From www.youtube.com
CSEC Chemistry Titrations and calculations YouTube Titration And Calculations Titration calculations generally involve this equation: Find the number of moles of acid. Moles of acid = 0.05 x 25/1000. And are the volumes of the acid and base, respectively. You want to measure the volumes at the. The second example addresses a weak acid titration requiring equilibrium calculations. At the equivalence point in a neutralization, the moles of acid. Titration And Calculations.
From www.youtube.com
Calculations with titrations 3 YouTube Titration And Calculations Titration calculations generally involve this equation: And are the volumes of the acid and base, respectively. Moles of acid = 0.05 x 25/1000. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. At the equivalence point in a neutralization, the. Titration And Calculations.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Titration And Calculations Moles of acid = concentration x volume in dm 3. You want to measure the volumes at the. Moles of acid = 0.05 x 25/1000. Calculate the concentration of the sodium hydroxide solution. The second example addresses a weak acid titration requiring equilibrium calculations. Find the number of moles of acid. Is the molarity of the acid, while is the. Titration And Calculations.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration And Calculations Moles of acid = 0.05 x 25/1000. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Moles of acid = concentration x volume in dm 3. Titration calculations generally involve this equation: You want to measure the volumes at the.. Titration And Calculations.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration And Calculations Calculate the concentration of the sodium hydroxide solution. You want to measure the volumes at the. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The. Titration And Calculations.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration And Calculations A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. Calculate the concentration of the sodium hydroxide solution. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Moles of acid = 0.05 x 25/1000. Is the molarity of. Titration And Calculations.
From mungfali.com
Acid Base Titration Calculation Titration And Calculations Moles of acid = concentration x volume in dm 3. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. And are the volumes of the acid. Titration And Calculations.
From yojkglpsgc.blogspot.com
How To Calculate Initial Concentration From Titration Curve Volume of Titration And Calculations Find the number of moles of acid. Calculate the concentration of the sodium hydroxide solution. Moles of acid = concentration x volume in dm 3. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. A titration is a volumetric technique in which a solution of one. Titration And Calculations.
From mavink.com
Acid Base Titration Calculation Titration And Calculations Titration calculations generally involve this equation: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. And are the volumes of the acid and base, respectively. Moles of acid. Titration And Calculations.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration Titration And Calculations Is the molarity of the acid, while is the molarity of the base. Find the number of moles of acid. Titration calculations generally involve this equation: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. A titration curve is a. Titration And Calculations.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration And Calculations Calculate the concentration of the sodium hydroxide solution. You want to measure the volumes at the. Moles of acid = concentration x volume in dm 3. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration curve is a plot of the concentration of the analyte at a. Titration And Calculations.
From theedge.com.hk
Chemistry How To Titration The Edge Titration And Calculations A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. The second example addresses a weak acid titration requiring equilibrium calculations. Titration calculations generally involve this equation: At the equivalence point in a neutralization, the moles of acid are equal to. Titration And Calculations.
From www.youtube.com
GCSE Chemistry Titration calculations worked examples YouTube Titration And Calculations Titration calculations generally involve this equation: A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. You want to measure the volumes at the. And are the volumes of the acid and base, respectively. Moles of acid = 0.05 x 25/1000. A titration is a volumetric technique. Titration And Calculations.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration And Calculations Titration calculations generally involve this equation: Is the molarity of the acid, while is the molarity of the base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. At the equivalence point in a neutralization, the moles of acid are. Titration And Calculations.
From www.youtube.com
pH titration curve calculations for weak acid strong base YouTube Titration And Calculations Moles of acid = 0.05 x 25/1000. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration calculations generally involve this equation: You want to measure the volumes at the. Moles of acid = concentration x volume in dm 3. Find the number of moles of acid. At the. Titration And Calculations.
From www.myxxgirl.com
Titration Calculations Example Video Chemistry Ck My XXX Hot Girl Titration And Calculations And are the volumes of the acid and base, respectively. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Find the number of moles of acid. Moles of. Titration And Calculations.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration And Calculations The second example addresses a weak acid titration requiring equilibrium calculations. Calculate the concentration of the sodium hydroxide solution. Moles of acid = 0.05 x 25/1000. Titration calculations generally involve this equation: You want to measure the volumes at the. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually. Titration And Calculations.
From docworksheet.com
Gcse Titration Calculations Worksheet Titration And Calculations And are the volumes of the acid and base, respectively. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Find the number of moles of acid. The second example addresses a weak acid titration requiring equilibrium calculations. Moles of acid = concentration x volume in dm 3. Titration calculations. Titration And Calculations.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration And Calculations Is the molarity of the acid, while is the molarity of the base. And are the volumes of the acid and base, respectively. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Find the number of moles of acid. Moles of acid = concentration x volume in dm 3.. Titration And Calculations.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration And Calculations The second example addresses a weak acid titration requiring equilibrium calculations. Titration calculations generally involve this equation: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Moles of acid = 0.05 x 25/1000. At the equivalence point in a neutralization, the moles of acid are equal to the moles. Titration And Calculations.