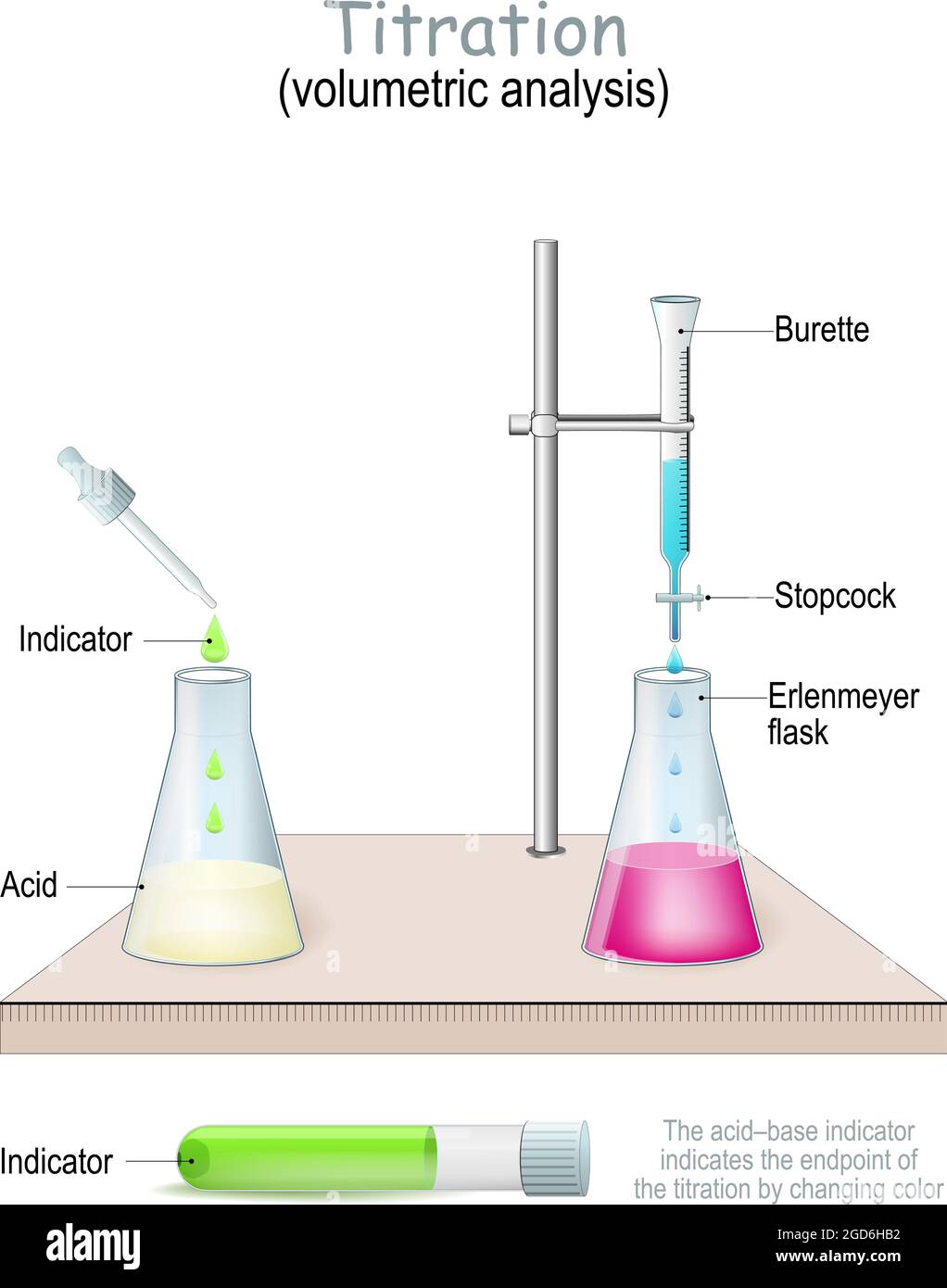
Titration Method Examples . Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. 10.0 ml of this solution is transferred to a. Find out how to choose an indicator, prepare the solutions, and calculate the. Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. Learn how to perform and interpret titration. Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution.
from ar.inspiredpencil.com
Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Find out how to choose an indicator, prepare the solutions, and calculate the. Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. 10.0 ml of this solution is transferred to a. Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. Learn how to perform and interpret titration.
Titration Diagram
Titration Method Examples Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Learn how to perform and interpret titration. Find out how to choose an indicator, prepare the solutions, and calculate the. 10.0 ml of this solution is transferred to a. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle Titration Method Examples 10.0 ml of this solution is transferred to a. Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. Find out how to choose an indicator, prepare the solutions, and calculate the. Titration is a chemical technique to calculate the concentration of an. Titration Method Examples.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Method Examples Find out how to choose an indicator, prepare the solutions, and calculate the. Learn how to perform and interpret titration. Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of. Titration Method Examples.
From augensternjiang.github.io
Chemistry Experiments and important reactions —— Common experiments Titration Method Examples Learn how to perform and interpret titration. 10.0 ml of this solution is transferred to a. Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. Find out how to choose an indicator, prepare the solutions, and calculate the. Titration is a technique to determine the concentration of. Titration Method Examples.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Method Examples Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. Learn how to perform and interpret titration. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. Titration is a technique to determine the concentration of an unknown solution. Titration Method Examples.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Method Examples Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. Learn how to perform and interpret titration. Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. Redox titration is an. Titration Method Examples.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Method Examples Find out how to choose an indicator, prepare the solutions, and calculate the. Learn how to perform and interpret titration. Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. 10.0 ml of this solution is transferred to a. Titration is a quantitative and volumetric technique to determine the unknown. Titration Method Examples.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Method Examples Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Find out how to choose an indicator, prepare the solutions, and calculate the. Titration is a. Titration Method Examples.
From www.science-revision.co.uk
Titrations Titration Method Examples Find out how to choose an indicator, prepare the solutions, and calculate the. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. Learn how to perform and interpret titration. Titration. Titration Method Examples.
From www.slideshare.net
Acid base titration (1) Titration Method Examples Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. 10.0 ml of this solution is transferred to a. Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. Find out. Titration Method Examples.
From bazaemmalyman.blogspot.com
Back Titration Method Emma Lyman Titration Method Examples Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. Learn how to perform and interpret titration. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. 10.0 ml of this solution is transferred to a. Titration is a technique to. Titration Method Examples.
From www.youtube.com
GCSE Chemistry Titration calculations worked examples YouTube Titration Method Examples 10.0 ml of this solution is transferred to a. Find out how to choose an indicator, prepare the solutions, and calculate the. Learn how to perform and interpret titration. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Titration is a quantitative and volumetric technique to determine. Titration Method Examples.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration Method Examples Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. Find out how to choose an indicator, prepare the solutions, and calculate the. Learn how to perform and interpret. Titration Method Examples.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Method Examples Find out how to choose an indicator, prepare the solutions, and calculate the. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. 10.0 ml of this solution is. Titration Method Examples.
From vasadylanpeake.blogspot.com
Back Titration Method Dylan Peake Titration Method Examples Learn how to perform and interpret titration. 10.0 ml of this solution is transferred to a. Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. Find out how to choose an indicator, prepare the solutions, and calculate the. A titration is a laboratory technique to measure the. Titration Method Examples.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Method Examples Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. 10.0 ml of this solution is transferred to a. Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. Learn how. Titration Method Examples.
From www.youtube.com
Precipitation Titrations Principle Mohr’s method Volhard’s Titration Method Examples Learn how to perform and interpret titration. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. Find out how to choose an indicator, prepare the solutions, and calculate. Titration Method Examples.
From quizizz.com
Titration Method Chemistry Quiz Quizizz Titration Method Examples 10.0 ml of this solution is transferred to a. Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. Learn how to perform and interpret titration. Find out how to choose an indicator, prepare the solutions, and calculate the. Titration is a technique. Titration Method Examples.
From www.researchgate.net
Representative example of conductometric titration from CNC batch 3 Titration Method Examples Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. Learn how to perform and interpret titration. Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. Titration is a technique to determine. Titration Method Examples.
From www.hotzxgirl.com
Titration Procedure Pdf Hot Sex Picture Titration Method Examples Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. 10.0 ml of this solution is transferred to a. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Learn how to perform and interpret titration. Titration is. Titration Method Examples.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Method Examples Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. A titration is a laboratory technique to measure the molar concentration. Titration Method Examples.
From revisechemistry.uk
Acidalkali titration edexcel Core Practical revisechemistry.uk Titration Method Examples Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Learn how to perform and interpret titration. Find out how to. Titration Method Examples.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Method Examples 10.0 ml of this solution is transferred to a. Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. Titration is a chemical. Titration Method Examples.
From studylib.net
KarlFischer Titration the method for determining water Titration Method Examples Find out how to choose an indicator, prepare the solutions, and calculate the. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. 10.0 ml of this solution is transferred to. Titration Method Examples.
From quizizz.com
Titrations Chemistry Quiz Quizizz Titration Method Examples Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Find out how to choose an indicator, prepare the solutions, and calculate the. Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence. Titration Method Examples.
From mirjamglessmer.com
Measuring the concentration of dissolved oxygen in sea water Part 3 Titration Method Examples Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. Learn how to perform and interpret titration. Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. Titration is a quantitative and volumetric technique to determine the unknown. Titration Method Examples.
From hubpages.com
Different Methods of Measuring Drug Potency, Concentration, Efficacy Titration Method Examples Learn how to perform and interpret titration. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Find out how to choose an indicator, prepare the solutions, and calculate the. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard. Titration Method Examples.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Method Examples 10.0 ml of this solution is transferred to a. Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Titration is a quantitative and volumetric technique to determine. Titration Method Examples.
From ar.inspiredpencil.com
Titration Diagram Titration Method Examples Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. A titration is a laboratory technique to measure the molar concentration of an unknown solution using. Titration Method Examples.
From chemistnotes.com
Precipitation Titration Principle, Types, and 5 Reliable Applications Titration Method Examples Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Find out how to choose an indicator, prepare the solutions, and. Titration Method Examples.
From idealpost.co.uk
Purpose And Important Types Of Titration Titration Method Examples Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard. Titration Method Examples.
From tukioka-clinic.com
️ Acid base titration problems with answers pdf. Eleventh grade Lesson Titration Method Examples Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. Learn how to perform and interpret titration. Find out how to choose an indicator, prepare the solutions, and calculate the. Titration is a chemical technique to calculate the concentration of an analyte in. Titration Method Examples.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts Titration Method Examples 10.0 ml of this solution is transferred to a. Titration is a quantitative and volumetric technique to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator. Find out how to choose an indicator, prepare the solutions, and calculate the. A titration is a laboratory technique to measure the molar concentration. Titration Method Examples.
From mavink.com
Titration Labeled Titration Method Examples Redox titration is an intriguing technique that delves into the transfer of electrons between two species in a chemical reaction. Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. Find out how to choose an indicator, prepare the solutions, and calculate the. Learn how to perform and. Titration Method Examples.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Method Examples Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another concentration until a. Redox titration is an intriguing technique that delves into the transfer of electrons between two species. Titration Method Examples.
From mavink.com
Titration Procedure Titration Method Examples Titration is a chemical technique to calculate the concentration of an analyte in a mixture by reacting it with a standard solution. Learn how to perform and interpret titration. A titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. Titration is a technique to determine the concentration of an unknown solution. Titration Method Examples.