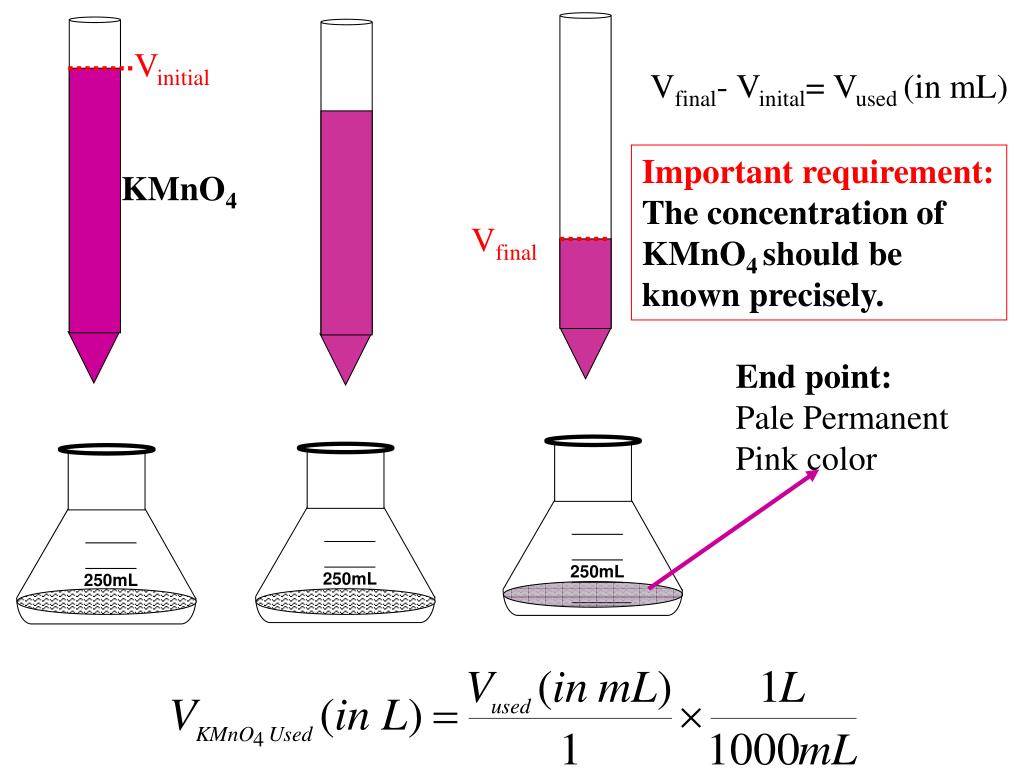
Analysis By Redox Titration Lab Answers . Redox titration is a laboratory technique for determining the concentration of a given analyte by initiating a redox reaction. Solution, given your previous answer and the fact that 25.00 ml were used? Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. To standardize a \(\ce{kio3}\) solution using a redox titration. Redox titration is the type of titration based on redox reaction between the analyte and titrant. Divide the following reaction into half. To compare your results for the. Determine the number of moles of fe2+ in solution. To analyze an unknown and commercial product for vitamin c content via titration. You will be trying to find the %. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the titrant. In this experiment, you will conduct redox titrations using a standardized permanganate solution. Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. Our titration was performed in an acidic solution.
from www.slideserve.com
You will be trying to find the %. Solution, given your previous answer and the fact that 25.00 ml were used? Our titration was performed in an acidic solution. Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. In this experiment, you will conduct redox titrations using a standardized permanganate solution. To standardize a \(\ce{kio3}\) solution using a redox titration. Determine the number of moles of fe2+ in solution. Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. Redox titration is a laboratory technique for determining the concentration of a given analyte by initiating a redox reaction. Redox titration is the type of titration based on redox reaction between the analyte and titrant.
PPT REDOX TITRATION PowerPoint Presentation ID431911
Analysis By Redox Titration Lab Answers Solution, given your previous answer and the fact that 25.00 ml were used? Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. You will be trying to find the %. To standardize a \(\ce{kio3}\) solution using a redox titration. To compare your results for the. Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. To analyze an unknown and commercial product for vitamin c content via titration. Solution, given your previous answer and the fact that 25.00 ml were used? Redox titration is the type of titration based on redox reaction between the analyte and titrant. Determine the number of moles of fe2+ in solution. Our titration was performed in an acidic solution. Divide the following reaction into half. Redox titration is a laboratory technique for determining the concentration of a given analyte by initiating a redox reaction. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the titrant. In this experiment, you will conduct redox titrations using a standardized permanganate solution.
From rileighrobertson2017.weebly.com
Permanganate Titration Rileigh Robertson Analysis By Redox Titration Lab Answers You will be trying to find the %. Divide the following reaction into half. To compare your results for the. To standardize a \(\ce{kio3}\) solution using a redox titration. Determine the number of moles of fe2+ in solution. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the titrant.. Analysis By Redox Titration Lab Answers.
From studylib.net
Redox Titration Analysis By Redox Titration Lab Answers To standardize a \(\ce{kio3}\) solution using a redox titration. Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. To compare your results for the. Solution, given your previous answer and the fact that 25.00 ml were used? In this experiment, you will conduct redox titrations using a standardized permanganate solution. You will be trying to. Analysis By Redox Titration Lab Answers.
From www.chegg.com
Solved So I'm doing a redox titration of vitamin c lab. Analysis By Redox Titration Lab Answers Solution, given your previous answer and the fact that 25.00 ml were used? To analyze an unknown and commercial product for vitamin c content via titration. Redox titration is the type of titration based on redox reaction between the analyte and titrant. Our titration was performed in an acidic solution. Redox titration is a laboratory technique for determining the concentration. Analysis By Redox Titration Lab Answers.
From www.youtube.com
Analysis of bleach redox titration lab YouTube Analysis By Redox Titration Lab Answers Redox titration is the type of titration based on redox reaction between the analyte and titrant. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the titrant. Determine the number of moles of fe2+ in solution. Our titration was performed in an acidic solution. To standardize a \(\ce{kio3}\) solution. Analysis By Redox Titration Lab Answers.
From www.youtube.com
REDOX REACTIONS AS THE BASIS FOR TITRATIONS YouTube Analysis By Redox Titration Lab Answers To standardize a \(\ce{kio3}\) solution using a redox titration. In this experiment, you will conduct redox titrations using a standardized permanganate solution. Redox titration is a laboratory technique for determining the concentration of a given analyte by initiating a redox reaction. To analyze an unknown and commercial product for vitamin c content via titration. Use stoichiometry to calculate the number. Analysis By Redox Titration Lab Answers.
From www.studypool.com
SOLUTION Redox titration lab sheet Studypool Analysis By Redox Titration Lab Answers To standardize a \(\ce{kio3}\) solution using a redox titration. To compare your results for the. Divide the following reaction into half. Our titration was performed in an acidic solution. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the titrant. Redox titration is the type of titration based on. Analysis By Redox Titration Lab Answers.
From www.numerade.com
REPORT SHEET EXPERIMENT Titration of Acids and Bases A. Analysis of an Analysis By Redox Titration Lab Answers You will be trying to find the %. Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. Solution, given your previous answer and the fact that 25.00 ml were used? Our titration was performed in an acidic solution. Redox titration is. Analysis By Redox Titration Lab Answers.
From www.chegg.com
Solved Experiment 9 Determination of Iron by Redox Analysis By Redox Titration Lab Answers Redox titration is the type of titration based on redox reaction between the analyte and titrant. Our titration was performed in an acidic solution. To analyze an unknown and commercial product for vitamin c content via titration. To standardize a \(\ce{kio3}\) solution using a redox titration. To compare your results for the. Redox titration is a laboratory technique for determining. Analysis By Redox Titration Lab Answers.
From www.scribd.com
Titration Lab Redox Titration Redox Analysis By Redox Titration Lab Answers Our titration was performed in an acidic solution. Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. In this experiment, you will conduct redox titrations using a standardized permanganate solution. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the titrant. Solution, given your. Analysis By Redox Titration Lab Answers.
From www.studypool.com
SOLUTION Lab 7 redox titration final Studypool Analysis By Redox Titration Lab Answers Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. Solution, given your previous answer and the fact that 25.00 ml were used? You will be trying to find the %. Redox titration is the type of titration based on redox reaction between the analyte and titrant. Our titration was performed in an acidic solution. Determine. Analysis By Redox Titration Lab Answers.
From www.coursehero.com
[Solved] calculate from your titration results the moles of vitamin C Analysis By Redox Titration Lab Answers In this experiment, you will conduct redox titrations using a standardized permanganate solution. To compare your results for the. You will be trying to find the %. Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. Determine the number of moles of fe2+ in solution. Divide the following reaction into half. To standardize a \(\ce{kio3}\). Analysis By Redox Titration Lab Answers.
From studylib.net
Redox Titration Potassium Permanganate and Sodium Oxalate Analysis By Redox Titration Lab Answers Our titration was performed in an acidic solution. Redox titration is the type of titration based on redox reaction between the analyte and titrant. In this experiment, you will conduct redox titrations using a standardized permanganate solution. Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. Redox titration is a laboratory method of determining the. Analysis By Redox Titration Lab Answers.
From www.studocu.com
Redox+Titration 101 redox lab Name ________Abigail Bondurant Date Analysis By Redox Titration Lab Answers Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. Our titration was performed in an acidic solution. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the titrant. You will be trying to find the %. Redox titration is a laboratory technique for determining. Analysis By Redox Titration Lab Answers.
From www.studocu.com
AP Redox Titration Lab Jan 2022 Name Analysis By Redox Titration Lab Answers Solution, given your previous answer and the fact that 25.00 ml were used? To compare your results for the. Redox titration is a laboratory technique for determining the concentration of a given analyte by initiating a redox reaction. In this experiment, you will conduct redox titrations using a standardized permanganate solution. Redox titration is the type of titration based on. Analysis By Redox Titration Lab Answers.
From mungfali.com
Acid Base Titration Lab Analysis By Redox Titration Lab Answers To standardize a \(\ce{kio3}\) solution using a redox titration. Solution, given your previous answer and the fact that 25.00 ml were used? In this experiment, you will conduct redox titrations using a standardized permanganate solution. You will be trying to find the %. Divide the following reaction into half. Our titration was performed in an acidic solution. Redox titration is. Analysis By Redox Titration Lab Answers.
From ar.inspiredpencil.com
Titration Analysis By Redox Titration Lab Answers Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. Redox titration is the type of titration based on redox reaction between the analyte and titrant. Redox titration is a laboratory method of determining the concentration of a given analyte by causing. Analysis By Redox Titration Lab Answers.
From www.chegg.com
Solved REPORT SHEET OxidationReduction Titrations II Analysis By Redox Titration Lab Answers Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. Redox titration is the type of titration based on redox reaction between the analyte and titrant. To compare your results for the. Solution, given your previous answer and the fact that 25.00 ml were used? To analyze an unknown and commercial product for vitamin c content. Analysis By Redox Titration Lab Answers.
From www.vrogue.co
Redox Titration Lab Docx Introduction Analytical Titr vrogue.co Analysis By Redox Titration Lab Answers Determine the number of moles of fe2+ in solution. In this experiment, you will conduct redox titrations using a standardized permanganate solution. Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. To compare your results for the. To analyze an unknown and commercial product for vitamin c content via titration. You will be trying to. Analysis By Redox Titration Lab Answers.
From www.chegg.com
Solved Chem 1 Lab Redox Titration and Determination of Fe. Analysis By Redox Titration Lab Answers You will be trying to find the %. To analyze an unknown and commercial product for vitamin c content via titration. Redox titration is the type of titration based on redox reaction between the analyte and titrant. Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. Solution, given your previous answer and the fact that. Analysis By Redox Titration Lab Answers.
From www.studocu.com
Redox Titration Lab Sheet Name ________________ Date Analysis By Redox Titration Lab Answers To compare your results for the. Solution, given your previous answer and the fact that 25.00 ml were used? Our titration was performed in an acidic solution. Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. Redox titration is a laboratory technique for determining the concentration of a given analyte by initiating a redox reaction.. Analysis By Redox Titration Lab Answers.
From www.studypool.com
SOLUTION Redox titration lab sheet Studypool Analysis By Redox Titration Lab Answers Our titration was performed in an acidic solution. You will be trying to find the %. Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. Redox titration is the type of titration based on redox reaction between the analyte and titrant. To standardize a \(\ce{kio3}\) solution using a redox titration. Divide the following reaction into. Analysis By Redox Titration Lab Answers.
From www.studocu.com
Redox Titration Lab The Determination of Iron (II) by Redox Titration Analysis By Redox Titration Lab Answers Redox titration is the type of titration based on redox reaction between the analyte and titrant. To standardize a \(\ce{kio3}\) solution using a redox titration. Divide the following reaction into half. Our titration was performed in an acidic solution. To analyze an unknown and commercial product for vitamin c content via titration. Another common analysis for iron is titration of. Analysis By Redox Titration Lab Answers.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation ID431911 Analysis By Redox Titration Lab Answers Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the titrant. To standardize a \(\ce{kio3}\) solution using a redox titration. Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. Solution, given your previous answer and the fact that 25.00 ml were used? Divide the. Analysis By Redox Titration Lab Answers.
From pradeepsirpharmacy.blogspot.com
Redox Titrations Analysis By Redox Titration Lab Answers Redox titration is a laboratory technique for determining the concentration of a given analyte by initiating a redox reaction. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the titrant. Solution, given your previous answer and the fact that 25.00 ml were used? To standardize a \(\ce{kio3}\) solution using. Analysis By Redox Titration Lab Answers.
From www.chegg.com
Solved Lab 5 Redox Titration and the Oxidizing Power of Analysis By Redox Titration Lab Answers Our titration was performed in an acidic solution. To analyze an unknown and commercial product for vitamin c content via titration. Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. In this experiment, you will conduct redox titrations using a standardized permanganate solution. You will be trying to find the %. Redox titration is a. Analysis By Redox Titration Lab Answers.
From melanie-chapter.blogspot.com
Titration Of Acids And Bases Lab Report Experiment 20 Answers 45+ Pages Analysis By Redox Titration Lab Answers In this experiment, you will conduct redox titrations using a standardized permanganate solution. Divide the following reaction into half. Solution, given your previous answer and the fact that 25.00 ml were used? Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. To compare your results for the. Redox titration is a laboratory method of determining. Analysis By Redox Titration Lab Answers.
From www.chegg.com
Solved OxidationReduction Titrations II analysis of Bleach Analysis By Redox Titration Lab Answers To standardize a \(\ce{kio3}\) solution using a redox titration. You will be trying to find the %. Solution, given your previous answer and the fact that 25.00 ml were used? Redox titration is the type of titration based on redox reaction between the analyte and titrant. Divide the following reaction into half. Redox titration is a laboratory method of determining. Analysis By Redox Titration Lab Answers.
From www.chegg.com
Redox Titration Analysis of Bleach lab report Analysis By Redox Titration Lab Answers To analyze an unknown and commercial product for vitamin c content via titration. Our titration was performed in an acidic solution. Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. In this experiment, you will conduct redox titrations using a standardized permanganate solution. To compare your results for the. Determine the number of moles of. Analysis By Redox Titration Lab Answers.
From www.scribd.com
Chemsheets A2 041 (Redox Titrations) Redox Titration Analysis By Redox Titration Lab Answers Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. Redox titration is the type of titration based on redox reaction between the analyte and titrant. Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. Determine the number of moles of fe2+ in solution. Solution, given your previous answer and the. Analysis By Redox Titration Lab Answers.
From www.vrogue.co
Titration Lab Redox Pdf Titration Chemistry vrogue.co Analysis By Redox Titration Lab Answers Redox titration is a laboratory technique for determining the concentration of a given analyte by initiating a redox reaction. You will be trying to find the %. Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. Divide the following reaction into half. Solution, given your previous answer and the fact that 25.00 ml were used?. Analysis By Redox Titration Lab Answers.
From www.studocu.com
Chemical Analysis by Redox Titration Questions 1. Use the method to Analysis By Redox Titration Lab Answers You will be trying to find the %. To standardize a \(\ce{kio3}\) solution using a redox titration. Redox titration is a laboratory technique for determining the concentration of a given analyte by initiating a redox reaction. Solution, given your previous answer and the fact that 25.00 ml were used? Another common analysis for iron is titration of iron (ii) with. Analysis By Redox Titration Lab Answers.
From www.numerade.com
SOLVED Text Report Sheet OxidationReduction Titrations Determination Analysis By Redox Titration Lab Answers Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. Redox titration is a laboratory technique for determining the concentration of a given analyte by initiating a redox reaction. Our titration was performed in an acidic solution. You will be trying to find the %. Redox titration is the type of titration based on redox reaction. Analysis By Redox Titration Lab Answers.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation, free download Analysis By Redox Titration Lab Answers You will be trying to find the %. Use stoichiometry to calculate the number of moles of mno4− used to oxidize the. To compare your results for the. Another common analysis for iron is titration of iron (ii) with potassium dichromate, k2cr2o7. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox. Analysis By Redox Titration Lab Answers.
From www.chegg.com
Lab 8 Analysis of Vinegar by Titration This page Analysis By Redox Titration Lab Answers To standardize a \(\ce{kio3}\) solution using a redox titration. Our titration was performed in an acidic solution. In this experiment, you will conduct redox titrations using a standardized permanganate solution. Divide the following reaction into half. Redox titration is the type of titration based on redox reaction between the analyte and titrant. Another common analysis for iron is titration of. Analysis By Redox Titration Lab Answers.
From www.scribd.com
Lower 6 Lab 4 Redox Titration Titration Redox Analysis By Redox Titration Lab Answers To standardize a \(\ce{kio3}\) solution using a redox titration. Divide the following reaction into half. To compare your results for the. You will be trying to find the %. In this experiment, you will conduct redox titrations using a standardized permanganate solution. Solution, given your previous answer and the fact that 25.00 ml were used? Redox titration is a laboratory. Analysis By Redox Titration Lab Answers.