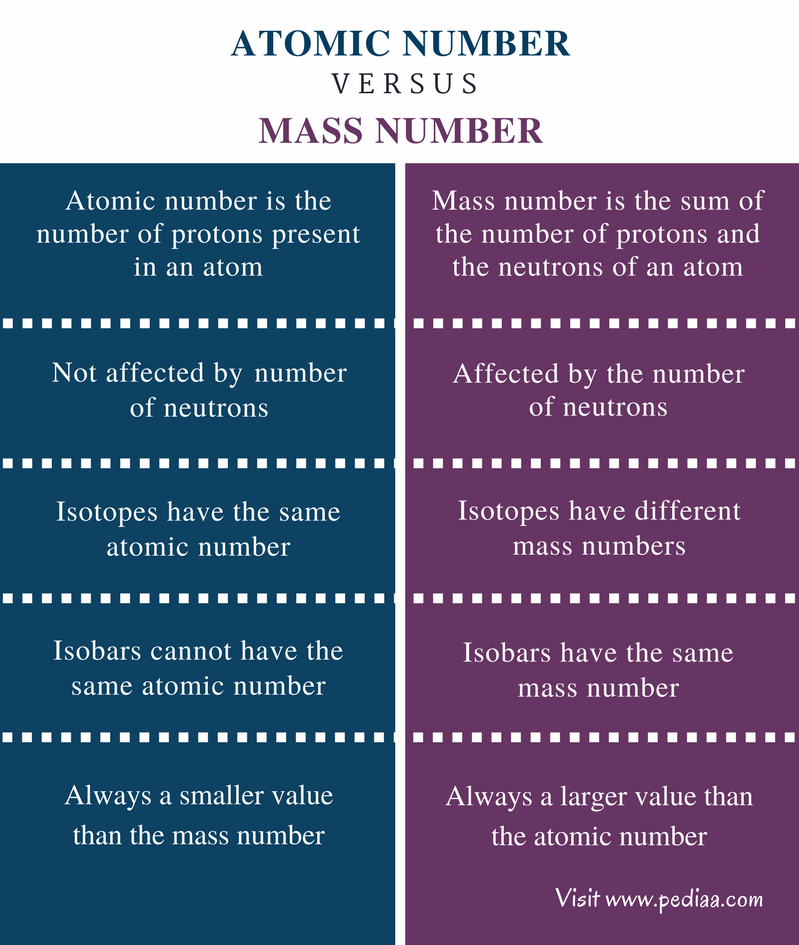
What Is Number Mass . Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a. Consider the table below, which shows data from the first six. For example, chlorine ( 37 17 cl) has a mass number of 37 and an atomic number of 17. This means that its nucleus contains 17. Contrast this with the atomic number, which is simply the number of protons. How can the mass number be calculated? The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. In other words, it is the sum of the number of nucleons in an atom. The mass number (represented by the letter a) is defined as the total number of protons and neutrons in an atom. The mass number of an atom is its total number of protons and neutrons. Well, the mass number of an atom is simply the number of massive, nuclear. Atoms of different elements usually have.
from pediaa.com
Well, the mass number of an atom is simply the number of massive, nuclear. Mass number is often denoted using a capital letter a. Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom. For example, chlorine ( 37 17 cl) has a mass number of 37 and an atomic number of 17. The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. How can the mass number be calculated? Consider the table below, which shows data from the first six. In other words, it is the sum of the number of nucleons in an atom. Contrast this with the atomic number, which is simply the number of protons. The mass number of an atom is its total number of protons and neutrons.
Difference Between Atomic Number and Mass Number Definition
What Is Number Mass Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number of an atom is its total number of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. How can the mass number be calculated? For example, chlorine ( 37 17 cl) has a mass number of 37 and an atomic number of 17. Well, the mass number of an atom is simply the number of massive, nuclear. Atoms of different elements usually have. Contrast this with the atomic number, which is simply the number of protons. Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom. The mass number (represented by the letter a) is defined as the total number of protons and neutrons in an atom. Consider the table below, which shows data from the first six. The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. This means that its nucleus contains 17.
From www.youtube.com
Atomic Number & Atomic Mass (Mass Number) amu, Moles, Grams, Molar What Is Number Mass Contrast this with the atomic number, which is simply the number of protons. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. This means that its nucleus contains 17. For example, chlorine ( 37 17 cl) has a mass number of 37 and an atomic number of. What Is Number Mass.
From www.youtube.com
Atomic Number & Mass Number Properties of Matter Chemistry What Is Number Mass Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. For example, chlorine ( 37 17 cl) has a mass number of 37 and an. What Is Number Mass.
From www.youtube.com
Atomic Number and Mass Number.mov YouTube What Is Number Mass The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. The mass number of an atom is its total number of protons and neutrons. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Well,. What Is Number Mass.
From chemistryskills.blogspot.com
Atomic Number Mass Number and Atomic Mass Unit What Is Number Mass Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom. Well, the mass number of an atom is simply the number of massive, nuclear. The mass number of an atom is its total number of protons and neutrons. How can the mass number be calculated? Atoms of different elements. What Is Number Mass.
From ppt-online.org
Atomic number, Mass number and Isotopes презентация онлайн What Is Number Mass The mass number (represented by the letter a) is defined as the total number of protons and neutrons in an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number of an atom is its total number of protons and neutrons. In other words,. What Is Number Mass.
From printablestarlitoamuqe.z21.web.core.windows.net
Moles And Avogadro's Number Formula What Is Number Mass How can the mass number be calculated? The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. For example, chlorine ( 37 17 cl) has a mass number of 37 and an atomic number of 17. Mass number, in nuclear physics, the sum of the numbers of. What Is Number Mass.
From pediaa.com
Difference Between Mass Number and Atomic Mass What Is Number Mass How can the mass number be calculated? Well, the mass number of an atom is simply the number of massive, nuclear. Mass number is often denoted using a capital letter a. The mass number (represented by the letter a) is defined as the total number of protons and neutrons in an atom. In other words, it is the sum of. What Is Number Mass.
From www.youtube.com
Atomic Number & Mass Number Science for CDS Exam CDS 2024 Science What Is Number Mass In other words, it is the sum of the number of nucleons in an atom. Consider the table below, which shows data from the first six. Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom. How can the mass number be calculated? The sum of the number of. What Is Number Mass.
From periodictable.me
Modern Periodic Table of Elements with Names and Symbols What Is Number Mass Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. How can the mass number be calculated? In other words, it is the sum of. What Is Number Mass.
From pediaa.com
Difference Between Mass Number and Atomic Mass What Is Number Mass Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom. Mass number is often denoted using a capital letter a. The mass number (represented by the letter a) is defined as the total number of protons and neutrons in an atom. This means that its nucleus contains 17. Consider. What Is Number Mass.
From reviewhomedecor.co
Periodic Table With Names And Atomic Mass Number Valency Review Home What Is Number Mass The mass number of an atom is its total number of protons and neutrons. Contrast this with the atomic number, which is simply the number of protons. Mass number is often denoted using a capital letter a. Well, the mass number of an atom is simply the number of massive, nuclear. For example, chlorine ( 37 17 cl) has a. What Is Number Mass.
From www.quia.com
Quia Atoms What Is Number Mass In other words, it is the sum of the number of nucleons in an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element.. What Is Number Mass.
From pediaa.com
Difference Between Mass Number and Atomic Mass What Is Number Mass This means that its nucleus contains 17. The mass number of an atom is its total number of protons and neutrons. How can the mass number be calculated? Contrast this with the atomic number, which is simply the number of protons. In other words, it is the sum of the number of nucleons in an atom. The sum of the. What Is Number Mass.
From elchoroukhost.net
Atomic Number Periodic Table Definition Elcho Table What Is Number Mass Atoms of different elements usually have. Consider the table below, which shows data from the first six. In other words, it is the sum of the number of nucleons in an atom. Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom. This means that its nucleus contains 17.. What Is Number Mass.
From www.slideserve.com
PPT The Structure of an Atom PowerPoint Presentation, free download What Is Number Mass Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. This means that its nucleus contains 17. Atoms of different elements usually have. Mass number is often denoted using a capital letter a. The sum of the number of protons and neutrons present in its nucleus is called. What Is Number Mass.
From www.slideserve.com
PPT 4.3 How Atoms Differ PowerPoint Presentation, free download ID What Is Number Mass This means that its nucleus contains 17. The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. The mass number of an atom is its total number of protons and neutrons. Atoms of different elements usually have. Consider the table below, which shows data from the first. What Is Number Mass.
From chemcafe.net
Why is it a mass number? ChemCafe — science, chemistry and physics What Is Number Mass Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom. Contrast this with the atomic number, which is simply the number of protons. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Consider the table below,. What Is Number Mass.
From sciencenotes.org
What Is an Atomic Number? Definition and Examples What Is Number Mass This means that its nucleus contains 17. Atoms of different elements usually have. The mass number of an atom is its total number of protons and neutrons. For example, chlorine ( 37 17 cl) has a mass number of 37 and an atomic number of 17. Well, the mass number of an atom is simply the number of massive, nuclear.. What Is Number Mass.
From ar.inspiredpencil.com
Mass And Atomic Mass Number What Is Number Mass Consider the table below, which shows data from the first six. This means that its nucleus contains 17. Contrast this with the atomic number, which is simply the number of protons. The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. Mass number is an integer (whole. What Is Number Mass.
From pediaa.com
Difference Between Atomic Number and Mass Number Definition What Is Number Mass Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom. Consider the table below, which shows data from the first six. Atoms of different elements usually have. In other words, it is the sum of the number of nucleons in an atom. For example, chlorine ( 37 17 cl). What Is Number Mass.
From mungfali.com
Periodic Table With Names And Atomic Mass What Is Number Mass The mass number (represented by the letter a) is defined as the total number of protons and neutrons in an atom. How can the mass number be calculated? Well, the mass number of an atom is simply the number of massive, nuclear. Consider the table below, which shows data from the first six. The mass number of an atom is. What Is Number Mass.
From syatillakmk.blogspot.com
SimplyChemistry C1 1.2PROTON NUMBER, MASS NUMBER, IONS & ISOTOPES What Is Number Mass How can the mass number be calculated? For example, chlorine ( 37 17 cl) has a mass number of 37 and an atomic number of 17. The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. Mass number is often denoted using a capital letter a. Contrast. What Is Number Mass.
From sciencenotes.org
Periodic Table with Atomic Mass What Is Number Mass The mass number (represented by the letter a) is defined as the total number of protons and neutrons in an atom. How can the mass number be calculated? The mass number of an atom is its total number of protons and neutrons. Well, the mass number of an atom is simply the number of massive, nuclear. For example, chlorine (. What Is Number Mass.
From www.examboard.in
Atomic Mass and Atomic Number of Elements (Periodic Table) What Is Number Mass Mass number is often denoted using a capital letter a. Consider the table below, which shows data from the first six. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. For example, chlorine ( 37 17 cl) has a mass number of 37 and an atomic number. What Is Number Mass.
From inspiritvr.com
Atomic Mass Unit Study Guide Inspirit What Is Number Mass Mass number is often denoted using a capital letter a. In other words, it is the sum of the number of nucleons in an atom. This means that its nucleus contains 17. Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom. The mass number (represented by the letter. What Is Number Mass.
From periodic-table-11.blogspot.com
PERIODIC TABLE MASS NUMBER LOCATED Periodic Table What Is Number Mass Consider the table below, which shows data from the first six. In other words, it is the sum of the number of nucleons in an atom. The mass number (represented by the letter a) is defined as the total number of protons and neutrons in an atom. Contrast this with the atomic number, which is simply the number of protons.. What Is Number Mass.
From ar.inspiredpencil.com
Magnesium Atomic Number And Mass What Is Number Mass Atoms of different elements usually have. Contrast this with the atomic number, which is simply the number of protons. Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom. Well, the mass number of an atom is simply the number of massive, nuclear. The mass number of an atom. What Is Number Mass.
From www.youtube.com
How To calculate mass number mass number calculation Part 1 YouTube What Is Number Mass Consider the table below, which shows data from the first six. Atoms of different elements usually have. The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. Well, the mass number of an atom is simply the number of massive, nuclear. The mass number of an atom. What Is Number Mass.
From www.medicowesome.com
Medicowesome Atomic number, mass number mnemonic for chemistry What Is Number Mass In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have. Contrast this with the atomic number, which is simply the number of protons. For example, chlorine ( 37 17 cl) has a mass number of 37 and an atomic number of 17. How can the mass number be calculated?. What Is Number Mass.
From www.vrogue.co
Periodic Table With Names And Atomic Mass And Number vrogue.co What Is Number Mass In other words, it is the sum of the number of nucleons in an atom. Consider the table below, which shows data from the first six. Mass number is often denoted using a capital letter a. The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. How. What Is Number Mass.
From www.thoughtco.com
Difference Between Atomic Mass and Mass Number What Is Number Mass Well, the mass number of an atom is simply the number of massive, nuclear. Mass number is often denoted using a capital letter a. The sum of the number of protons and neutrons present in its nucleus is called mass number of an atom of an element. How can the mass number be calculated? Atoms of different elements usually have.. What Is Number Mass.
From www.pinterest.com
WEEK 13 SCIENCE Atomic Number and Mass Number Chemistry the virtual What Is Number Mass The mass number of an atom is its total number of protons and neutrons. Well, the mass number of an atom is simply the number of massive, nuclear. How can the mass number be calculated? Atoms of different elements usually have. The mass number (represented by the letter a) is defined as the total number of protons and neutrons in. What Is Number Mass.
From ar.inspiredpencil.com
Mass Number Example What Is Number Mass The mass number of an atom is its total number of protons and neutrons. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Consider the table below, which shows data from the first six. Atoms of different elements usually have. How can the mass number be calculated?. What Is Number Mass.
From kason-blogduran.blogspot.com
What is Atomic Number What Is Number Mass Atoms of different elements usually have. For example, chlorine ( 37 17 cl) has a mass number of 37 and an atomic number of 17. In other words, it is the sum of the number of nucleons in an atom. This means that its nucleus contains 17. Mass number is an integer (whole number) equal to the sum of the. What Is Number Mass.
From www.sliderbase.com
Mass Number What Is Number Mass Well, the mass number of an atom is simply the number of massive, nuclear. Consider the table below, which shows data from the first six. How can the mass number be calculated? The mass number of an atom is its total number of protons and neutrons. Mass number is an integer (whole number) equal to the sum of the number. What Is Number Mass.