Zinc Outermost Electron Shell . it's easier to understand electron configuration and valence if you can actually see the electrons surrounding. electron shells the energy levels of the electrons within an atom. while zinc ions are electron acceptor that possess empty orbitals after losing their outermost electrons, leading. shells, subshells, and orbitals. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: in chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the distribution of outermost shell electrons, known as valence electrons, of organic molecules was observed for the first time. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal. zinc's location reflects the fact that is has a completely filled third electron shell. physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically. Orbitals most precise information typically. what is a valence orbital diagram?
from www.slideshare.net
it's easier to understand electron configuration and valence if you can actually see the electrons surrounding. The first shell (closest to the. the distribution of outermost shell electrons, known as valence electrons, of organic molecules was observed for the first time. Orbitals most precise information typically. zinc's location reflects the fact that is has a completely filled third electron shell. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically. what is a valence orbital diagram? physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons.
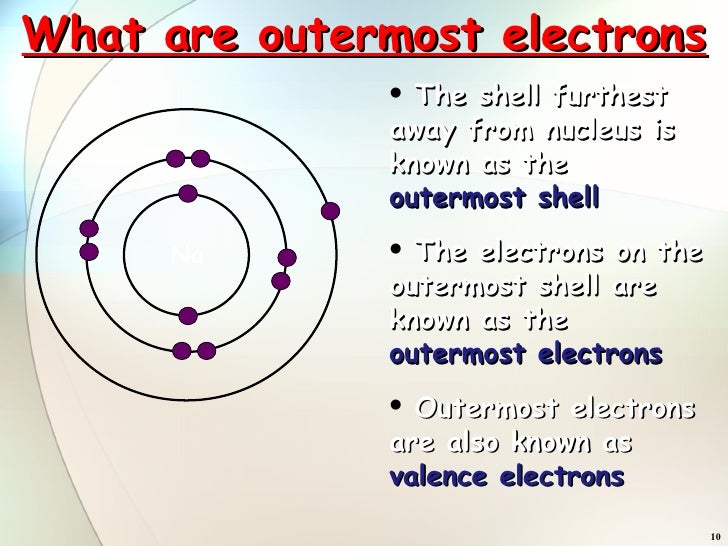
Structure Of Atoms Part 3
Zinc Outermost Electron Shell why does zinc lose electrons while its outermost configuration is 4$\rm{s}^2$ 3$\rm{d}^{10}$ both. shells, subshells, and orbitals. why does zinc lose electrons while its outermost configuration is 4$\rm{s}^2$ 3$\rm{d}^{10}$ both. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically. Orbitals most precise information typically. zinc's location reflects the fact that is has a completely filled third electron shell. electron shells the energy levels of the electrons within an atom. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal. while zinc ions are electron acceptor that possess empty orbitals after losing their outermost electrons, leading. the outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. the presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. an atom's ground state electron configuration describes how the electrons have distributed among the orbital shells. what is a valence orbital diagram? the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. it's easier to understand electron configuration and valence if you can actually see the electrons surrounding.
From brainly.com
What will oxygen likely do to complete its outermost shell, based on Zinc Outermost Electron Shell physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. it's easier to understand electron configuration and valence if you can actually see the electrons surrounding. Orbitals most precise information typically. the presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: . Zinc Outermost Electron Shell.
From material-properties.org
Zinc Periodic Table and Atomic Properties Zinc Outermost Electron Shell an atom's ground state electron configuration describes how the electrons have distributed among the orbital shells. in chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the. the distribution of electrons in shells in a zinc atom can be represented as follows: The first shell (closest to. Zinc Outermost Electron Shell.
From materiallibrarycagle.z13.web.core.windows.net
If Each Orbital Contain 3 Electrons Zinc Outermost Electron Shell while zinc ions are electron acceptor that possess empty orbitals after losing their outermost electrons, leading. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in. Zinc Outermost Electron Shell.
From www.focuskimia.com
General Chemistry Review Valence Electrons of The First Row Elements Zinc Outermost Electron Shell in chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the. the presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: shells, subshells, and orbitals. the distribution of electrons in shells in a zinc atom can be. Zinc Outermost Electron Shell.
From dxofdyyse.blob.core.windows.net
Zinc Number Of Unpaired Electrons at Bernadine Campbell blog Zinc Outermost Electron Shell the presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: zinc's location reflects the fact that is has a completely filled third electron shell. in chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the. physicists and. Zinc Outermost Electron Shell.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Zinc Outermost Electron Shell the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically. The first shell (closest to the. it's easier to understand electron configuration and valence if you can actually see the electrons surrounding. the. Zinc Outermost Electron Shell.
From manuallistaeschylus.z14.web.core.windows.net
What Do Electron Dot Diagrams Show Zinc Outermost Electron Shell while zinc ions are electron acceptor that possess empty orbitals after losing their outermost electrons, leading. what is a valence orbital diagram? The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically. Orbitals most precise information typically. the outermost orbital shell of an atom is called its valence shell, and the. Zinc Outermost Electron Shell.
From en.wikipedia.org
FileElectron shell 030 Zinc.svg Wikipedia, the free encyclopedia Zinc Outermost Electron Shell the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. what is a valence orbital diagram? Orbitals most precise information typically. the electrons occupying the outermost shell orbital(s) (highest value. Zinc Outermost Electron Shell.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Zinc Outermost Electron Shell The first shell (closest to the. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the distribution of outermost shell electrons, known as valence electrons, of organic molecules was observed for the first time. shells, subshells, and orbitals. the electrons occupying the outermost shell orbital(s) (highest value. Zinc Outermost Electron Shell.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Zinc Outermost Electron Shell the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal. the electrons occupying the outermost shell orbital(s) (highest value of n) are. Zinc Outermost Electron Shell.
From slideplayer.com
Chemical Bonding. ppt download Zinc Outermost Electron Shell an atom's ground state electron configuration describes how the electrons have distributed among the orbital shells. shells, subshells, and orbitals. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Zinc Outermost Electron Shell.
From www.nagwa.com
Question Video Identifying the Number of Electrons in the Outermost Zinc Outermost Electron Shell shells, subshells, and orbitals. in chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the. why does zinc lose electrons while its outermost configuration is 4$\rm{s}^2$ 3$\rm{d}^{10}$ both. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically. it's easier. Zinc Outermost Electron Shell.
From guidemuimeik.z14.web.core.windows.net
Orbital Diagrams Of All Elements Zinc Outermost Electron Shell the presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: The first shell (closest to the. while zinc ions are electron acceptor that possess empty orbitals after losing their outermost electrons, leading. the outermost orbital shell of an atom is called its valence shell, and the electrons in. Zinc Outermost Electron Shell.
From pixels.com
Scandium Electron Configuration Photograph by Zinc Outermost Electron Shell The first shell (closest to the. physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically. what is a valence orbital diagram? since it is the outermost (valence) electrons which are primarily involved in chemical. Zinc Outermost Electron Shell.
From www.alamy.com
Symbol and electron diagram for Zinc illustration Stock Vector Image Zinc Outermost Electron Shell the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the distribution of outermost shell electrons, known as valence electrons, of organic molecules was observed for the first time. the presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: . Zinc Outermost Electron Shell.
From manuallistranterism.z4.web.core.windows.net
Electron Distribution Diagram For Sodium Zinc Outermost Electron Shell Orbitals most precise information typically. physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. why does zinc lose electrons while its outermost configuration is 4$\rm{s}^2$ 3$\rm{d}^{10}$ both. shells, subshells, and orbitals. The first shell (closest to the. the distribution of outermost shell electrons, known as valence electrons, of organic. Zinc Outermost Electron Shell.
From www.slideserve.com
PPT Electrons and the Periodic Table PowerPoint Presentation, free Zinc Outermost Electron Shell the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically. since it is the outermost (valence) electrons which are primarily involved in chemical interactions between. shells, subshells, and orbitals. it's easier to. Zinc Outermost Electron Shell.
From www.nagwa.com
Question Video Determining the Number of Electrons in the Valence Zinc Outermost Electron Shell while zinc ions are electron acceptor that possess empty orbitals after losing their outermost electrons, leading. Orbitals most precise information typically. physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. why does zinc lose electrons while its outermost configuration is 4$\rm{s}^2$ 3$\rm{d}^{10}$ both. in chemistry and physics, valence electrons. Zinc Outermost Electron Shell.
From www.slideserve.com
PPT Chemistry for Bio 9 PowerPoint Presentation, free download ID Zinc Outermost Electron Shell while zinc ions are electron acceptor that possess empty orbitals after losing their outermost electrons, leading. an atom's ground state electron configuration describes how the electrons have distributed among the orbital shells. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. it's easier to understand electron configuration. Zinc Outermost Electron Shell.
From schematicfixgingles.z21.web.core.windows.net
Orbital Diagram For Oxygen Zinc Outermost Electron Shell Orbitals most precise information typically. what is a valence orbital diagram? it's easier to understand electron configuration and valence if you can actually see the electrons surrounding. the presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: the electron configurations of silicon (14 electrons), phosphorus (15 electrons),. Zinc Outermost Electron Shell.
From diagramdataconfusion.z22.web.core.windows.net
Electron Arrangement Diagram Zinc Outermost Electron Shell an atom's ground state electron configuration describes how the electrons have distributed among the orbital shells. shells, subshells, and orbitals. zinc's location reflects the fact that is has a completely filled third electron shell. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. in chemistry and. Zinc Outermost Electron Shell.
From www.sciencephoto.com
Zinc electron configuration Stock Image C029/5029 Science Photo Library Zinc Outermost Electron Shell why does zinc lose electrons while its outermost configuration is 4$\rm{s}^2$ 3$\rm{d}^{10}$ both. electron shells the energy levels of the electrons within an atom. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. The valence orbital diagram is a graphical representation of the electron configuration of an atom,. Zinc Outermost Electron Shell.
From www.vrogue.co
Electron Shells And Orbitals vrogue.co Zinc Outermost Electron Shell The first shell (closest to the. physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. zinc's location reflects the fact that is has a completely filled third electron shell. in chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the.. Zinc Outermost Electron Shell.
From www.collegesearch.in
Electronic Configuration of First 30 Elements Significance, 3 Rules Zinc Outermost Electron Shell while zinc ions are electron acceptor that possess empty orbitals after losing their outermost electrons, leading. Orbitals most precise information typically. shells, subshells, and orbitals. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. an atom's ground state electron configuration describes how the electrons have distributed among. Zinc Outermost Electron Shell.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Zinc Outermost Electron Shell the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the distribution of outermost shell electrons, known as valence electrons, of organic molecules was observed for the first time. why does zinc lose electrons while its outermost configuration is 4$\rm{s}^2$ 3$\rm{d}^{10}$ both. physicists and chemists use a standard. Zinc Outermost Electron Shell.
From www.slideshare.net
Structure Of Atoms Part 3 Zinc Outermost Electron Shell in chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the. while zinc ions are electron acceptor that possess empty orbitals after losing their outermost electrons, leading. physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. it's easier to. Zinc Outermost Electron Shell.
From periodictable.me
Valence+Electrons+The+electrons+on+the+outermost+shell+of+an+atom Zinc Outermost Electron Shell the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and. Zinc Outermost Electron Shell.
From brainly.in
Why valency of Scandium is 3 but in google electronic configuration is Zinc Outermost Electron Shell it's easier to understand electron configuration and valence if you can actually see the electrons surrounding. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively,. Zinc Outermost Electron Shell.
From brainly.in
an atom of an element has four electron in the outermost M shell what Zinc Outermost Electron Shell Orbitals most precise information typically. why does zinc lose electrons while its outermost configuration is 4$\rm{s}^2$ 3$\rm{d}^{10}$ both. it's easier to understand electron configuration and valence if you can actually see the electrons surrounding. The first shell (closest to the. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically. what. Zinc Outermost Electron Shell.
From exoroknts.blob.core.windows.net
Lead Outermost Electrons at Donnie Kell blog Zinc Outermost Electron Shell the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. zinc's location reflects the fact that is has a completely filled third electron shell. it's easier to understand electron configuration and valence if you can actually see the electrons surrounding. why does zinc lose electrons while its outermost. Zinc Outermost Electron Shell.
From www.slideshare.net
Structure Of Atoms Part 3 Zinc Outermost Electron Shell while zinc ions are electron acceptor that possess empty orbitals after losing their outermost electrons, leading. the outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. the distribution of outermost shell electrons, known as valence electrons, of organic molecules was observed for the first time.. Zinc Outermost Electron Shell.
From chemistrytalk.org
Electron Shells ChemTalk Zinc Outermost Electron Shell the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal. since it is the outermost (valence) electrons which are primarily involved in. Zinc Outermost Electron Shell.
From www.vedantu.com
An atom of an element has \\,4\\, electrons in the outermost \\,M Zinc Outermost Electron Shell The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically. the distribution of outermost shell electrons, known as valence electrons, of organic molecules was observed for the first time. physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. the presence of valence electrons can. Zinc Outermost Electron Shell.
From profliway.hubpages.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Zinc Outermost Electron Shell the distribution of outermost shell electrons, known as valence electrons, of organic molecules was observed for the first time. The first shell (closest to the. since it is the outermost (valence) electrons which are primarily involved in chemical interactions between. the presence of valence electrons can determine the element's chemical properties and whether it may bond with. Zinc Outermost Electron Shell.
From dxonyecim.blob.core.windows.net
Zn Electron Configuration Diagram at Beatrice Fitch blog Zinc Outermost Electron Shell the distribution of electrons in shells in a zinc atom can be represented as follows: the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. while zinc ions are electron. Zinc Outermost Electron Shell.