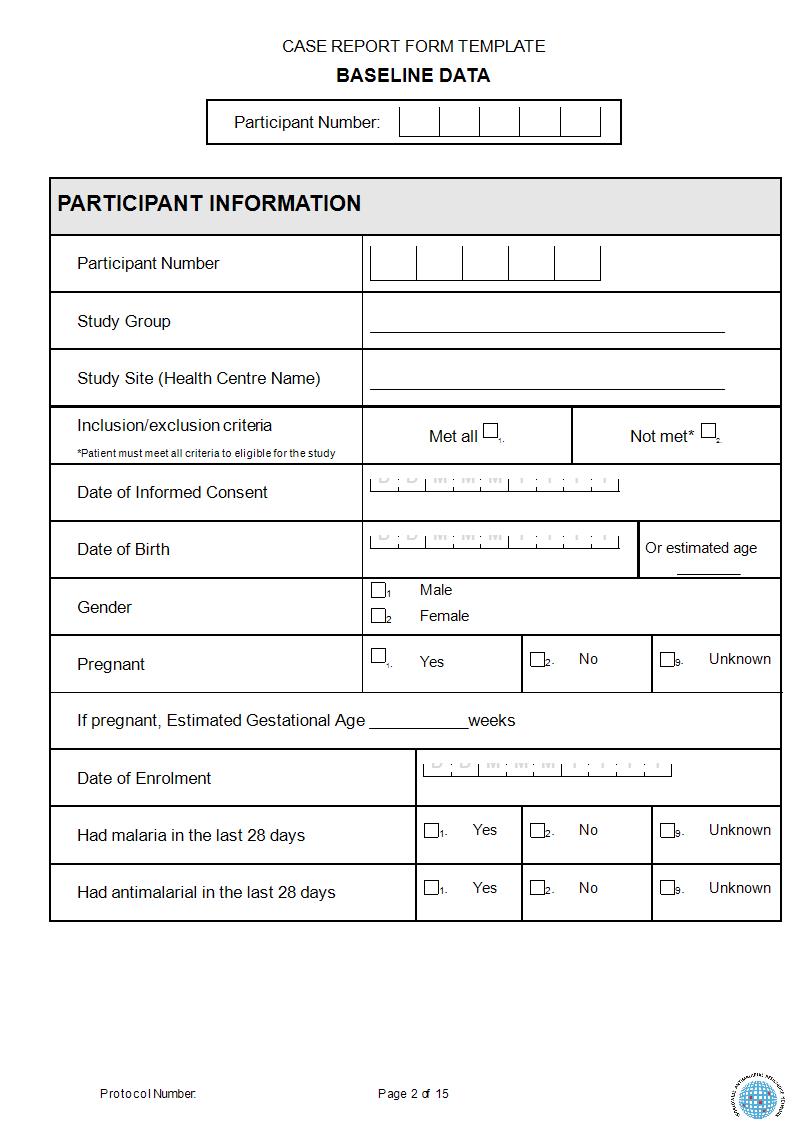
Case Report Requirements . Written informed consent for publication must be obtained from. This checklist is relevant to case reports and case series, and is based on the care. The structure of a case report usually comprises a. Consent for publication is a mandatory journal requirement for all case reports. Case reports should be short and focused, with a limited number of figures and references. Checklist for reporting a case report or case series. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base.
from www.atlanticcityaquarium.com
Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. Case reports should be short and focused, with a limited number of figures and references. Checklist for reporting a case report or case series. This checklist is relevant to case reports and case series, and is based on the care. Written informed consent for publication must be obtained from. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: Consent for publication is a mandatory journal requirement for all case reports. The structure of a case report usually comprises a.
Case Report Form Template Clinical Trials
Case Report Requirements Consent for publication is a mandatory journal requirement for all case reports. Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. The structure of a case report usually comprises a. Written informed consent for publication must be obtained from. Checklist for reporting a case report or case series. Case reports should be short and focused, with a limited number of figures and references. This checklist is relevant to case reports and case series, and is based on the care. Consent for publication is a mandatory journal requirement for all case reports. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated:
From www.researchgate.net
(PDF) Case report guidelines and informed consent Case Report Requirements Written informed consent for publication must be obtained from. Consent for publication is a mandatory journal requirement for all case reports. This checklist is relevant to case reports and case series, and is based on the care. Checklist for reporting a case report or case series. Case report, as a research design, describes important scientific observations that are encountered in. Case Report Requirements.
From criticalthinking.cloud
medical student case report journal Case Report Requirements Written informed consent for publication must be obtained from. Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: Case reports should be short and focused,. Case Report Requirements.
From med.cornell.libguides.com
Home Case Reports LibGuides at Weill Cornell Medical College Case Report Requirements Consent for publication is a mandatory journal requirement for all case reports. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: This checklist is relevant to case reports and case series, and is based on the care. Written informed consent for publication must be obtained from. Case. Case Report Requirements.
From www.semanticscholar.org
CARE guidelines for case reports explanation and elaboration document Case Report Requirements The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: This checklist is relevant to case reports and case series, and is based on the care. The structure of a case report usually comprises a. Checklist for reporting a case report or case series. Written informed consent for. Case Report Requirements.
From www.mathewsopenaccess.com
Author Guidelines Case Reports Open Access Journals Mathews Case Report Requirements The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: Checklist for reporting a case report or case series. The structure of a case report usually comprises a. Case reports should be short and focused, with a limited number of figures and references. Consent for publication is a. Case Report Requirements.
From www.sampletemplates.com
FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs Case Report Requirements This checklist is relevant to case reports and case series, and is based on the care. Checklist for reporting a case report or case series. Written informed consent for publication must be obtained from. Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. The structure of a. Case Report Requirements.
From studylib.net
CASE STUDY REPORT FORMAT GUIDELINE Case Report Requirements The structure of a case report usually comprises a. This checklist is relevant to case reports and case series, and is based on the care. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: Case report, as a research design, describes important scientific observations that are encountered. Case Report Requirements.
From www.sampletemplates.com
Sample Business Requirements Document 6+ Free Documents In PDF, Word Case Report Requirements This checklist is relevant to case reports and case series, and is based on the care. The structure of a case report usually comprises a. Case reports should be short and focused, with a limited number of figures and references. The various documents are grouped in three sections according to the stage of the trial during which they will normally. Case Report Requirements.
From www.researchgate.net
(PDF) How to write a case report? Guidelines for Internists Case Report Requirements The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: Consent for publication is a mandatory journal requirement for all case reports. This checklist is relevant to case reports and case series, and is based on the care. Case report, as a research design, describes important scientific observations. Case Report Requirements.
From www.pdffiller.com
Fillable Online SECTION 21 CASE REPORT GUIDELINES Fax Email Print Case Report Requirements Checklist for reporting a case report or case series. This checklist is relevant to case reports and case series, and is based on the care. Case reports should be short and focused, with a limited number of figures and references. The various documents are grouped in three sections according to the stage of the trial during which they will normally. Case Report Requirements.
From www.ijcrcentral.com
Information For Authors International Journal of Clinical Research Case Report Requirements Checklist for reporting a case report or case series. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: Written informed consent for publication must be obtained from. Consent for publication is a mandatory journal requirement for all case reports. This checklist is relevant to case reports and. Case Report Requirements.
From www.slideshare.net
Psychological assessment report Case Report Requirements Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. Written informed consent for publication must be obtained from. Checklist for reporting a case report or case series. The structure of a case report usually comprises a. This checklist is relevant to case reports and case series, and. Case Report Requirements.
From www.sohu.com
CARE报告指南:让病例报告更规范、透明、有据可循(二) 《病例报告撰写规范CARE解读》_Journal_期刊_Hedache Case Report Requirements Written informed consent for publication must be obtained from. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: Consent for publication is a mandatory journal requirement for all case reports. The structure of a case report usually comprises a. This checklist is relevant to case reports and. Case Report Requirements.
From www.samplestemplates.org
7+ Police Report Templates in Word PDF Free Formats Excel Word Case Report Requirements Consent for publication is a mandatory journal requirement for all case reports. Checklist for reporting a case report or case series. Written informed consent for publication must be obtained from. Case reports should be short and focused, with a limited number of figures and references. The various documents are grouped in three sections according to the stage of the trial. Case Report Requirements.
From www.allbusinesstemplates.com
Free Police Report Template Templates at Case Report Requirements Consent for publication is a mandatory journal requirement for all case reports. Checklist for reporting a case report or case series. Written informed consent for publication must be obtained from. Case reports should be short and focused, with a limited number of figures and references. Case report, as a research design, describes important scientific observations that are encountered in a. Case Report Requirements.
From www.scribd.com
Case Report Guidelines General Case Report Medical Diagnosis Case Report Requirements Checklist for reporting a case report or case series. This checklist is relevant to case reports and case series, and is based on the care. Consent for publication is a mandatory journal requirement for all case reports. The structure of a case report usually comprises a. Case report, as a research design, describes important scientific observations that are encountered in. Case Report Requirements.
From medicinewalls.blogspot.com
Case Report Internal Medicine Journal MedicineWalls Case Report Requirements Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. Case reports should be short and focused, with a limited number of figures and references. Written informed consent for publication must be obtained from. Checklist for reporting a case report or case series. This checklist is relevant to. Case Report Requirements.
From www.researchgate.net
(PDF) An overview of writing a case report Case Report Requirements Checklist for reporting a case report or case series. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: The structure of a case report usually comprises a. Written informed consent for publication must be obtained from. Case reports should be short and focused, with a limited number. Case Report Requirements.
From www.researchgate.net
(PDF) The CARE (CAse REport) guidelines and the standardization of case Case Report Requirements Case reports should be short and focused, with a limited number of figures and references. Checklist for reporting a case report or case series. The structure of a case report usually comprises a. Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. The various documents are grouped. Case Report Requirements.
From www.businessanalystlearnings.com
The 3Step Guide to Documenting Requirements with Use Cases — Business Case Report Requirements Consent for publication is a mandatory journal requirement for all case reports. This checklist is relevant to case reports and case series, and is based on the care. Checklist for reporting a case report or case series. Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. Case. Case Report Requirements.
From www.researchgate.net
CARE (CAse REport) checklist Download Scientific Diagram Case Report Requirements Checklist for reporting a case report or case series. Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. This checklist is relevant to case reports and case series, and is based on the care. The various documents are grouped in three sections according to the stage of. Case Report Requirements.
From documents.thegreenerleithsocial.org
Case Report Form Template Case Report Requirements Case reports should be short and focused, with a limited number of figures and references. The structure of a case report usually comprises a. Consent for publication is a mandatory journal requirement for all case reports. This checklist is relevant to case reports and case series, and is based on the care. Checklist for reporting a case report or case. Case Report Requirements.
From www.template.net
7+ Requirement Analysis Templates Word, Docs, PDF Case Report Requirements Written informed consent for publication must be obtained from. Consent for publication is a mandatory journal requirement for all case reports. Checklist for reporting a case report or case series. This checklist is relevant to case reports and case series, and is based on the care. The various documents are grouped in three sections according to the stage of the. Case Report Requirements.
From businesstemplateinspiration.blogspot.com
Case Report Form Template Case Report Requirements Case reports should be short and focused, with a limited number of figures and references. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. Consent. Case Report Requirements.
From www.aerzteblatt.de
Die Case Reporting (CARE) Guideline Case Report Requirements Case reports should be short and focused, with a limited number of figures and references. This checklist is relevant to case reports and case series, and is based on the care. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: The structure of a case report usually. Case Report Requirements.
From www.atlanticcityaquarium.com
Case Report Form Template Clinical Trials Case Report Requirements This checklist is relevant to case reports and case series, and is based on the care. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: The structure of a case report usually comprises a. Written informed consent for publication must be obtained from. Checklist for reporting a. Case Report Requirements.
From www.researchgate.net
(PDF) Guideline on writing a case report Case Report Requirements This checklist is relevant to case reports and case series, and is based on the care. Checklist for reporting a case report or case series. The structure of a case report usually comprises a. Written informed consent for publication must be obtained from. The various documents are grouped in three sections according to the stage of the trial during which. Case Report Requirements.
From www.researchgate.net
(PDF) CARE guidelines for case reports explanation and elaboration Case Report Requirements Consent for publication is a mandatory journal requirement for all case reports. Written informed consent for publication must be obtained from. Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. The structure of a case report usually comprises a. This checklist is relevant to case reports and. Case Report Requirements.
From mavink.com
Peri Care Checklist Printable Case Report Requirements The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: This checklist is relevant to case reports and case series, and is based on the care. Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base.. Case Report Requirements.
From www.semanticscholar.org
Table 2 from The SCARE 2020 Guideline Updating Consensus Surgical CAse Case Report Requirements Checklist for reporting a case report or case series. Written informed consent for publication must be obtained from. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: This checklist is relevant to case reports and case series, and is based on the care. The structure of a. Case Report Requirements.
From www.jacc.org
How to Write Your First Clinical Case Report JACC Case Reports Case Report Requirements Case reports should be short and focused, with a limited number of figures and references. The structure of a case report usually comprises a. Written informed consent for publication must be obtained from. This checklist is relevant to case reports and case series, and is based on the care. Case report, as a research design, describes important scientific observations that. Case Report Requirements.
From www.researchgate.net
CARE Criteria Case report studies. Download Table Case Report Requirements Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. Consent for publication is a mandatory journal requirement for all case reports. Case reports should be short and focused, with a limited number of figures and references. The various documents are grouped in three sections according to the. Case Report Requirements.
From www.researchgate.net
(PDF) How to write a case report for publication Case Report Requirements Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. Checklist for reporting a case report or case series. The structure of a case report usually comprises a. Case reports should be short and focused, with a limited number of figures and references. Consent for publication is a. Case Report Requirements.
From studylib.net
Howtowriteacasereport Case Report Requirements This checklist is relevant to case reports and case series, and is based on the care. Checklist for reporting a case report or case series. Case reports should be short and focused, with a limited number of figures and references. Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our. Case Report Requirements.
From www.smithchavezlaw.com
Reporting Requirements Template (12) TEMPLATES EXAMPLE TEMPLATES Case Report Requirements Case report, as a research design, describes important scientific observations that are encountered in a clinical setting to expand our knowledge base. Consent for publication is a mandatory journal requirement for all case reports. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated: Checklist for reporting a. Case Report Requirements.