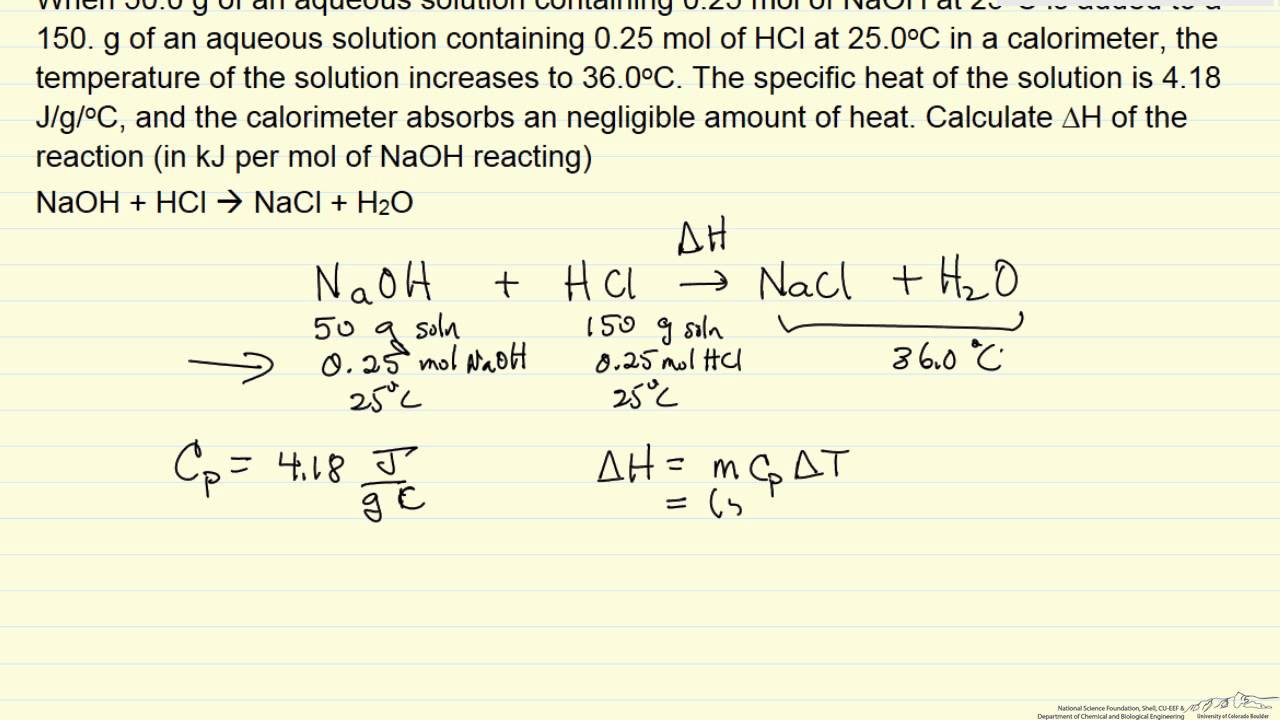
Calorimeter Specific Heat Lab . The specific heat has been calculated for many substances and can be. Specific heat can be defined as the amount of heat required (q) to raise the temperature of one gram of the substance by. Specific heat for selected substances. Measuring heat transfer with a calorimeter introduction. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. Identifying a metal by measuring specific heat. The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. The specific heat capacity is different for different substances, as can be seen in table 1. This lab will have two experiment parts. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. The density of water is. A 59.7 g piece of metal that had been submerged in boiling water was quickly transferred into 60.0 ml of water initially at 22.0 °c. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. The reservoir in a calorimeter used to study reactions occurring in. The specific heat of water has the relatively high value of 4.184 j/g⋅k.
from www.youtube.com
The density of water is. Specific heat for selected substances. The reservoir in a calorimeter used to study reactions occurring in. A 59.7 g piece of metal that had been submerged in boiling water was quickly transferred into 60.0 ml of water initially at 22.0 °c. The specific heat capacity is different for different substances, as can be seen in table 1. The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. The specific heat of water has the relatively high value of 4.184 j/g⋅k. Measuring heat transfer with a calorimeter introduction. Specific heat can be defined as the amount of heat required (q) to raise the temperature of one gram of the substance by. Identifying a metal by measuring specific heat.
Heat of Reaction from a Calorimeter (Example) YouTube
Calorimeter Specific Heat Lab Identifying a metal by measuring specific heat. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. A 59.7 g piece of metal that had been submerged in boiling water was quickly transferred into 60.0 ml of water initially at 22.0 °c. Identifying a metal by measuring specific heat. Specific heat for selected substances. Measuring heat transfer with a calorimeter introduction. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. Specific heat can be defined as the amount of heat required (q) to raise the temperature of one gram of the substance by. The specific heat of water has the relatively high value of 4.184 j/g⋅k. The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. The specific heat has been calculated for many substances and can be. The reservoir in a calorimeter used to study reactions occurring in. The density of water is. This lab will have two experiment parts. The specific heat capacity is different for different substances, as can be seen in table 1. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values.
From saylordotorg.github.io
Calorimetry Calorimeter Specific Heat Lab The density of water is. The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. Measuring heat transfer with a calorimeter introduction. The reservoir in a calorimeter used to study reactions occurring in. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. The specific heat capacity is. Calorimeter Specific Heat Lab.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Specific Heat Lab A 59.7 g piece of metal that had been submerged in boiling water was quickly transferred into 60.0 ml of water initially at 22.0 °c. Specific heat can be defined as the amount of heat required (q) to raise the temperature of one gram of the substance by. The specific heat capacity is different for different substances, as can be. Calorimeter Specific Heat Lab.
From fyogshfpe.blob.core.windows.net
Lab Calorimetry And Specific Heat Data Table at Russell Kelly blog Calorimeter Specific Heat Lab The specific heat has been calculated for many substances and can be. This lab will have two experiment parts. The specific heat capacity is different for different substances, as can be seen in table 1. The specific heat of water has the relatively high value of 4.184 j/g⋅k. The reservoir in a calorimeter used to study reactions occurring in. The. Calorimeter Specific Heat Lab.
From www.tessshebaylo.com
Equation For Constant Volume Calorimetry Tessshebaylo Calorimeter Specific Heat Lab A 59.7 g piece of metal that had been submerged in boiling water was quickly transferred into 60.0 ml of water initially at 22.0 °c. This lab will have two experiment parts. The density of water is. The specific heat of water has the relatively high value of 4.184 j/g⋅k. Since the solution is aqueous, we can proceed as if. Calorimeter Specific Heat Lab.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimeter Specific Heat Lab The specific heat capacity is different for different substances, as can be seen in table 1. The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. The specific heat of water has the relatively high value of 4.184 j/g⋅k. The specific heat has been calculated for many substances and can be. Specific heat for selected. Calorimeter Specific Heat Lab.
From ar.inspiredpencil.com
Specific Heat Lab Calorimeter Calorimeter Specific Heat Lab Specific heat for selected substances. Identifying a metal by measuring specific heat. The specific heat capacity is different for different substances, as can be seen in table 1. This lab will have two experiment parts. Measuring heat transfer with a calorimeter introduction. The specific heat has been calculated for many substances and can be. Specific heat can be defined as. Calorimeter Specific Heat Lab.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Specific Heat Lab This lab will have two experiment parts. The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. The specific heat capacity is different for different substances, as can be seen in table 1. Identifying a metal by measuring specific heat. A 59.7 g piece of metal that had been submerged in boiling water was quickly. Calorimeter Specific Heat Lab.
From www.scribd.com
Lab 07Specific Heat & Calorimetry.pdf Heat Temperature Calorimeter Specific Heat Lab This lab will have two experiment parts. Specific heat for selected substances. The specific heat has been calculated for many substances and can be. The specific heat capacity is different for different substances, as can be seen in table 1. The density of water is. The first is to determine the heat capacity of a calorimeter in a constant pressure. Calorimeter Specific Heat Lab.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Calorimeter Specific Heat Lab The reservoir in a calorimeter used to study reactions occurring in. The specific heat has been calculated for many substances and can be. Identifying a metal by measuring specific heat. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. Since the solution is aqueous, we can proceed as if it were water. Calorimeter Specific Heat Lab.
From www.studocu.com
Lab Calorimetry and Specific Heat SCIENCE LAB REPORT Please go to Calorimeter Specific Heat Lab The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. This lab will have two experiment parts. The specific heat of water has the relatively high value of 4.184 j/g⋅k. The reservoir in a calorimeter used to study reactions occurring in. Specific heat can be defined as the amount of heat required (q) to raise. Calorimeter Specific Heat Lab.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Calorimeter Specific Heat Lab The density of water is. The reservoir in a calorimeter used to study reactions occurring in. The specific heat of water has the relatively high value of 4.184 j/g⋅k. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. Specific heat can be defined as the amount of. Calorimeter Specific Heat Lab.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry Lab Sec Name Calorimeter Specific Heat Lab Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. Measuring heat transfer with a calorimeter introduction. Specific heat for selected substances. The specific heat of water has the relatively high value of. Calorimeter Specific Heat Lab.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry / 613 Lob Sec. Calorimeter Specific Heat Lab Measuring heat transfer with a calorimeter introduction. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. Specific heat for selected substances. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. The density of water is. The specific heat of. Calorimeter Specific Heat Lab.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Specific Heat Lab This lab will have two experiment parts. The specific heat of water has the relatively high value of 4.184 j/g⋅k. The specific heat has been calculated for many substances and can be. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. Identifying a metal by measuring specific. Calorimeter Specific Heat Lab.
From answerhappy.com
CALORIMETRY HEAT CAPACITY OF A CALORIMETER NTRODUCTION Lab Data Calorimeter Specific Heat Lab The reservoir in a calorimeter used to study reactions occurring in. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. A 59.7 g piece of metal that had been submerged in boiling water was quickly transferred into 60.0 ml of water initially at 22.0 °c. The density of water is approximately 1.0. Calorimeter Specific Heat Lab.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Specific Heat Lab The reservoir in a calorimeter used to study reactions occurring in. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. The density of water is. A 59.7 g piece of metal that had been submerged in boiling water was quickly transferred into 60.0 ml of water initially. Calorimeter Specific Heat Lab.
From ar.inspiredpencil.com
Specific Heat Lab Calorimeter Calorimeter Specific Heat Lab The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. The specific heat of water has the relatively high value of 4.184 j/g⋅k. This lab will have two experiment parts. Since the solution is aqueous, we can proceed. Calorimeter Specific Heat Lab.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimeter Specific Heat Lab Specific heat can be defined as the amount of heat required (q) to raise the temperature of one gram of the substance by. The specific heat has been calculated for many substances and can be. Specific heat for selected substances. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and. Calorimeter Specific Heat Lab.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Specific Heat Lab Specific heat can be defined as the amount of heat required (q) to raise the temperature of one gram of the substance by. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. The specific heat has been calculated for many substances and can be. The specific heat of water has the relatively. Calorimeter Specific Heat Lab.
From www.studocu.com
Calorimetry Lab Report Name Mahma Ahmed Partner Wala Naeem Student Calorimeter Specific Heat Lab Measuring heat transfer with a calorimeter introduction. The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. Specific heat can be defined as the amount of heat required (q) to raise the temperature of one gram of the substance by. Since the solution is aqueous, we can proceed as if it were water in terms. Calorimeter Specific Heat Lab.
From courses.lumenlearning.com
5.2 Calorimetry Chemistry Calorimeter Specific Heat Lab The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. The specific heat of water has the relatively high value of 4.184 j/g⋅k. The specific heat has been calculated for many substances and can be. Measuring heat transfer with a calorimeter introduction. Specific heat can be defined as the amount of heat required. Calorimeter Specific Heat Lab.
From users.highland.edu
Calorimetry Calorimeter Specific Heat Lab Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. Measuring heat transfer with a calorimeter introduction. Identifying a metal by measuring specific heat. The reservoir in a calorimeter used to study reactions occurring in. A 59.7 g piece of metal that had been submerged in boiling water. Calorimeter Specific Heat Lab.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Calorimeter Specific Heat Lab The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. Specific heat for selected substances. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. Specific heat can be defined as the amount of heat required (q) to raise the temperature of one. Calorimeter Specific Heat Lab.
From www.youtube.com
Specific Heat of Metal Sample Calorimetry Lab Problem solved YouTube Calorimeter Specific Heat Lab The specific heat capacity is different for different substances, as can be seen in table 1. Specific heat for selected substances. The specific heat has been calculated for many substances and can be. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. The specific heat of water. Calorimeter Specific Heat Lab.
From www.aliexpress.com
Calorimeter Specific Heat Experiment Physics Teaching Instrument Calorimeter Specific Heat Lab This lab will have two experiment parts. Measuring heat transfer with a calorimeter introduction. Specific heat can be defined as the amount of heat required (q) to raise the temperature of one gram of the substance by. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. Identifying. Calorimeter Specific Heat Lab.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards Calorimeter Specific Heat Lab The density of water is. Specific heat for selected substances. Measuring heat transfer with a calorimeter introduction. The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. The specific heat of water has the relatively high value of 4.184 j/g⋅k. Specific heat can be defined as the amount of heat required (q) to raise the. Calorimeter Specific Heat Lab.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimeter Specific Heat Lab Identifying a metal by measuring specific heat. The specific heat has been calculated for many substances and can be. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. A 59.7 g piece of metal that had been submerged in boiling water was quickly transferred into 60.0 ml of water initially at 22.0. Calorimeter Specific Heat Lab.
From www.youtube.com
Heat of Reaction from a Calorimeter (Example) YouTube Calorimeter Specific Heat Lab Specific heat can be defined as the amount of heat required (q) to raise the temperature of one gram of the substance by. Specific heat for selected substances. The reservoir in a calorimeter used to study reactions occurring in. The specific heat capacity is different for different substances, as can be seen in table 1. The first is to determine. Calorimeter Specific Heat Lab.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimeter Specific Heat Lab The reservoir in a calorimeter used to study reactions occurring in. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. The specific heat has been calculated for many substances and can be. The density of water is. This lab will have two experiment parts. The first is. Calorimeter Specific Heat Lab.
From www.thinkswap.com
Calorimetry Lab Report PHYS1006 Foundations of Physics Curtin Calorimeter Specific Heat Lab The reservoir in a calorimeter used to study reactions occurring in. Identifying a metal by measuring specific heat. A 59.7 g piece of metal that had been submerged in boiling water was quickly transferred into 60.0 ml of water initially at 22.0 °c. This lab will have two experiment parts. Specific heat can be defined as the amount of heat. Calorimeter Specific Heat Lab.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry Lab Sec Name Calorimeter Specific Heat Lab The specific heat has been calculated for many substances and can be. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. Specific heat for selected substances. The specific heat capacity is different for different substances, as can be seen in table 1. The density of water is.. Calorimeter Specific Heat Lab.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Calorimeter Specific Heat Lab Identifying a metal by measuring specific heat. Specific heat for selected substances. The specific heat has been calculated for many substances and can be. The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. Specific heat can be defined as the amount of heat required (q) to raise the temperature of one gram of the. Calorimeter Specific Heat Lab.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimeter Specific Heat Lab The specific heat has been calculated for many substances and can be. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. Identifying a metal by measuring specific heat. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. This lab. Calorimeter Specific Heat Lab.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Calorimeter Specific Heat Lab The density of water is. A 59.7 g piece of metal that had been submerged in boiling water was quickly transferred into 60.0 ml of water initially at 22.0 °c. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. The specific heat capacity is different for different. Calorimeter Specific Heat Lab.
From www.youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Calorimeter Specific Heat Lab The reservoir in a calorimeter used to study reactions occurring in. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. The density of water is approximately 1.0 g/ml, so 100.0 ml has a mass of. Identifying a metal by measuring specific heat. The density of water is.. Calorimeter Specific Heat Lab.