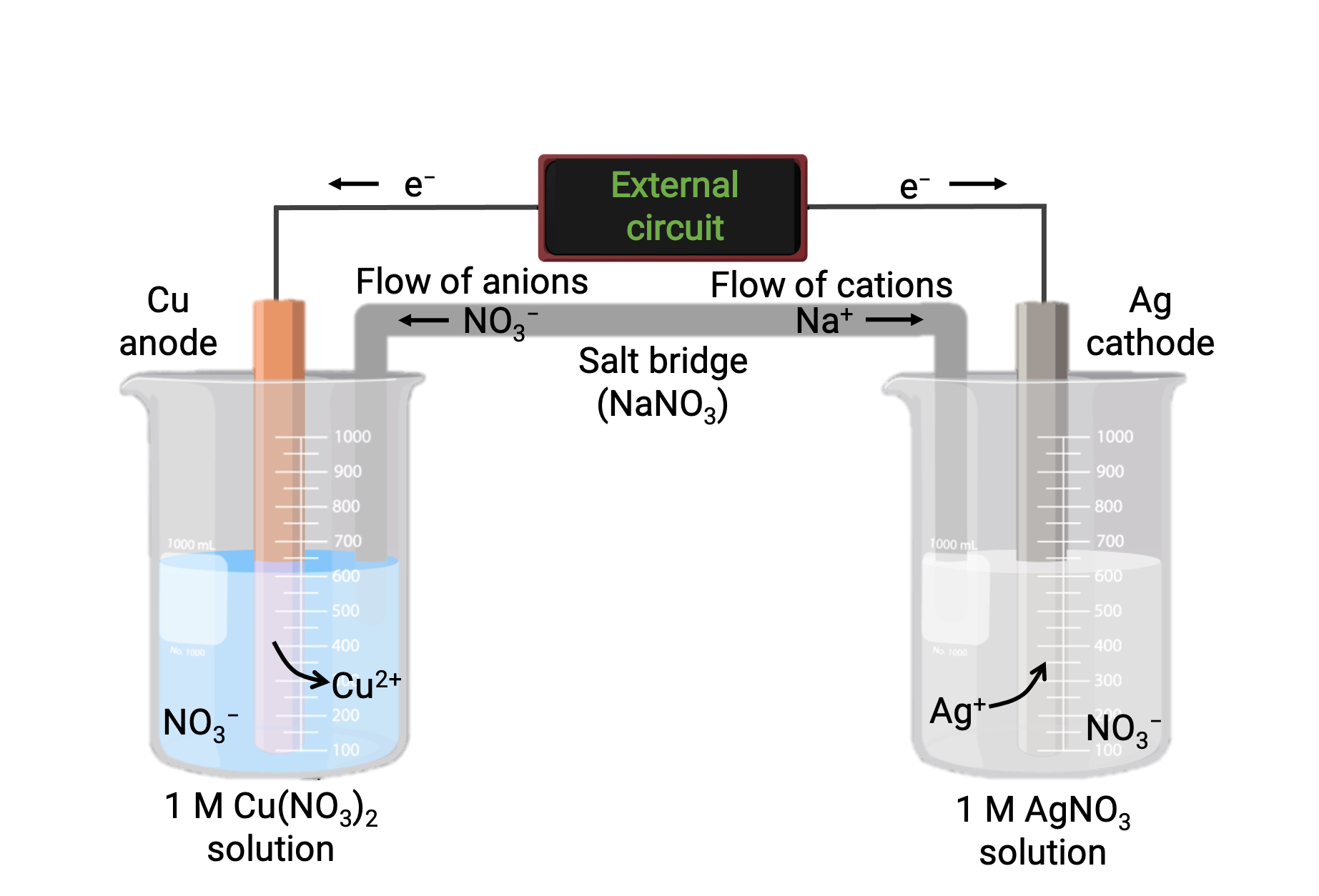
Galvanic Cell Cathode . A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. — electrons flow from the anode to the cathode: Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the.
from www.jove.com
A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. Left to right in the standard galvanic cell in the figure. — electrons flow from the anode to the cathode: in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a.
Voltaic/Galvanic Cells Principle, Components, Cell Notation (Video) JoVE
Galvanic Cell Cathode in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. Left to right in the standard galvanic cell in the figure. — electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a.
From www.freepik.com
Premium Photo Electrochemical cell or Galvanic cell. The Daniell cell Galvanic Cell Cathode — electrons flow from the anode to the cathode: Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to. Galvanic Cell Cathode.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Galvanic Cell Cathode by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. Left to right in the standard galvanic cell in the figure. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. — electrons flow from. Galvanic Cell Cathode.
From biochemreview.weebly.com
Galvanic cell BIOChemReview Galvanic Cell Cathode — electrons flow from the anode to the cathode: A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. in a galvanic cell, current is produced when electrons flow externally through the. Galvanic Cell Cathode.
From mavink.com
Galvanic Cell Cathode Anode Galvanic Cell Cathode by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. Left to right in the standard galvanic cell in the figure. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. — electrons flow from the anode to the cathode: in a. Galvanic Cell Cathode.
From www.chegg.com
Solved b) On the galvanic cell, label the anode, cathode and Galvanic Cell Cathode Left to right in the standard galvanic cell in the figure. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. — electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. in a. Galvanic Cell Cathode.
From www.pinterest.com
What Is a Galvanic Cell? Galvanic cell, Electrochemical cell, Science Galvanic Cell Cathode Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. — electrons flow from the anode to the cathode: in a. Galvanic Cell Cathode.
From userdatarheumatics.z21.web.core.windows.net
Simple Cell Diagram Chemistry Galvanic Cell Cathode in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. — electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. Left to right in the standard. Galvanic Cell Cathode.
From saylordotorg.github.io
Commercial Galvanic Cells Galvanic Cell Cathode in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the.. Galvanic Cell Cathode.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Galvanic Cell Cathode in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. — electrons flow from. Galvanic Cell Cathode.
From www.shutterstock.com
Voltaic Galvanic Cell Copper Cathode Magnesium Stock Vector (Royalty Galvanic Cell Cathode Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. — electrons flow from the anode to the cathode: in a. Galvanic Cell Cathode.
From www.youtube.com
Determining the Anode/Cathode for a Galvanic Cell Test Boost for AP Galvanic Cell Cathode — electrons flow from the anode to the cathode: A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. by definition, the anode of an electrochemical cell is. Galvanic Cell Cathode.
From mavink.com
Galvanic Cell Cathode Anode Galvanic Cell Cathode Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. in a galvanic cell, current is produced when electrons flow externally through. Galvanic Cell Cathode.
From courses.lumenlearning.com
Galvanic Cells Chemistry Galvanic Cell Cathode A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. — electrons flow from the anode to the cathode: Left to right in the standard galvanic cell in the figure. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of. Galvanic Cell Cathode.
From studybreathings.z21.web.core.windows.net
How Do Electrons Flow In A Galvanic Cell Galvanic Cell Cathode by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. Left to right in the standard galvanic cell in the figure. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. — electrons flow from the anode to the cathode: in a. Galvanic Cell Cathode.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo Galvanic Cell Cathode by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. — electrons flow from the anode to the cathode: Left to right in the standard galvanic cell in the figure. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. in a. Galvanic Cell Cathode.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Galvanic Cell Cathode by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a.. Galvanic Cell Cathode.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 Galvanic Cell Cathode Left to right in the standard galvanic cell in the figure. — electrons flow from the anode to the cathode: A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. in a. Galvanic Cell Cathode.
From stock.adobe.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Galvanic Cell Cathode A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. — electrons flow from the anode to the cathode: Left to right in the standard galvanic cell in the. Galvanic Cell Cathode.
From www.tes.com
Galvanic Cells, Anode, Cathode, Oxidation, Reduction, Grade 12 Galvanic Cell Cathode Left to right in the standard galvanic cell in the figure. — electrons flow from the anode to the cathode: A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of. Galvanic Cell Cathode.
From studybreathings.z21.web.core.windows.net
How Do Electrons Flow In A Galvanic Cell Galvanic Cell Cathode in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. Left to right in the standard galvanic cell in the figure. — electrons flow from the anode to the cathode: A cathode is defined as an electrode through which positive/negative electric charge flows out of/into. Galvanic Cell Cathode.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle Galvanic Cell Cathode Left to right in the standard galvanic cell in the figure. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. in a galvanic cell, current is produced when electrons flow externally through. Galvanic Cell Cathode.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID235007 Galvanic Cell Cathode in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the.. Galvanic Cell Cathode.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download Galvanic Cell Cathode in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. — electrons flow from the anode to the cathode: Left to right in the standard galvanic cell in the. Galvanic Cell Cathode.
From courses.lumenlearning.com
Galvanic Cells Chemistry Atoms First Galvanic Cell Cathode — electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. Left to right in the standard galvanic cell in the figure. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. in a. Galvanic Cell Cathode.
From wps.prenhall.com
Media Portfolio Galvanic Cell Cathode Left to right in the standard galvanic cell in the figure. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. — electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. in a. Galvanic Cell Cathode.
From electrochemistryh5t34.blogspot.com
We Love Electrochemistry Galvanic Cell Galvanic Cell Cathode A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. — electrons flow from the anode to the cathode: Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. in a. Galvanic Cell Cathode.
From quizturbinates.z21.web.core.windows.net
How Does Galvanic Cell Work Galvanic Cell Cathode Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. — electrons flow from the anode to the cathode: in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to. Galvanic Cell Cathode.
From stock.adobe.com
Vector scientific illustration of the electrolysis processes. Set of Galvanic Cell Cathode by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. — electrons flow from the anode to the cathode: in a galvanic cell, current is produced when electrons flow externally through the. Galvanic Cell Cathode.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic Galvanic Cell Cathode — electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. Left to right in the standard galvanic cell in the figure. in a. Galvanic Cell Cathode.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation (Video) JoVE Galvanic Cell Cathode A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the.. Galvanic Cell Cathode.
From www.shutterstock.com
Diagrama de células electroquímicas. Célula galvánica vector de stock Galvanic Cell Cathode in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. — electrons flow from the anode to the cathode: Left to right in the standard galvanic cell in the figure. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into. Galvanic Cell Cathode.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Galvanic Cell Cathode — electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. Left to right in the standard galvanic cell in the figure. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. in a. Galvanic Cell Cathode.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Galvanic Cell Cathode Left to right in the standard galvanic cell in the figure. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. — electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation. Galvanic Cell Cathode.
From mungfali.com
Galvanic Cell Anode And Cathode Galvanic Cell Cathode A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. — electrons flow from the anode to the cathode: in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a. by definition, the anode of an electrochemical cell is. Galvanic Cell Cathode.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Galvanic Cell Cathode Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a. in a galvanic cell, current is produced when electrons flow externally through. Galvanic Cell Cathode.