Standard Enthalpy Of Formation C2H2 . Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. standard enthalpies of formation. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. Δh °f δ h ° f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. All standard state, 25 °c and 1 bar (written to 1 decimal place). For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c.
from www.chegg.com
Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. Δh °f δ h ° f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in. All standard state, 25 °c and 1 bar (written to 1 decimal place). For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. standard enthalpies of formation. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation of any element in its standard state is zero by definition.
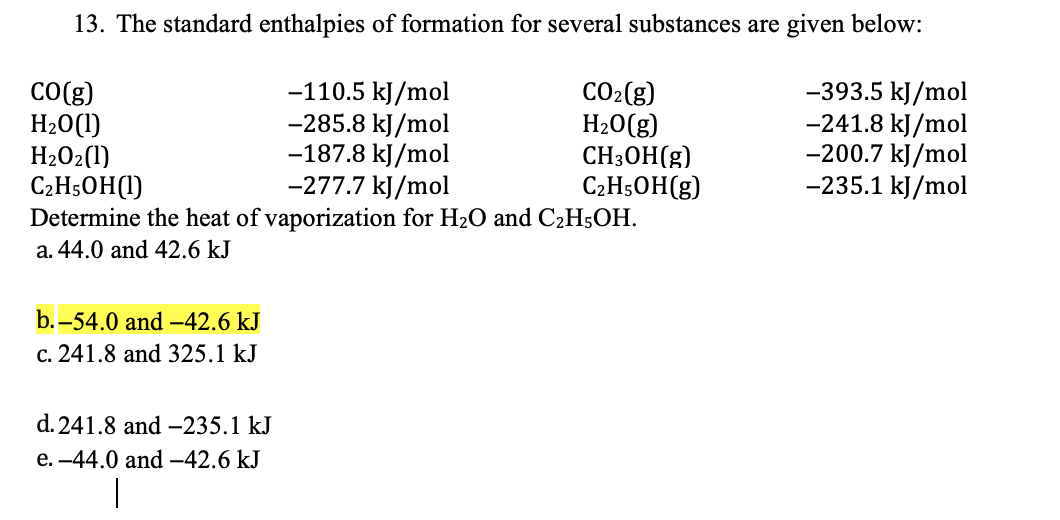
Solved 13. The standard enthalpies of formation for several
Standard Enthalpy Of Formation C2H2 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of any element in its standard state is zero by definition. All standard state, 25 °c and 1 bar (written to 1 decimal place). standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Δh °f δ h ° f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of.
From www.chegg.com
Solved 1. The enthalpy of formation ΔH∗, of acetylene (C2H2) Standard Enthalpy Of Formation C2H2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation of any element in its standard state is zero by definition. Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: the standard enthalpy of formation is a measure of the energy released. Standard Enthalpy Of Formation C2H2.
From www.numerade.com
SOLVED Calculate the enthalpy change for the combustion of acetylene Standard Enthalpy Of Formation C2H2 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: the standard enthalpy of formation of any element in its standard state is zero. Standard Enthalpy Of Formation C2H2.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation C2H2 the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: For example, although oxygen can exist as. Standard Enthalpy Of Formation C2H2.
From www.toppr.com
The molar enthalpies of combustion of C2H2(g) , Cl (graphite) and H2(g Standard Enthalpy Of Formation C2H2 Δh °f δ h ° f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. For example, although oxygen can exist as ozone (o 3. Standard Enthalpy Of Formation C2H2.
From www.numerade.com
SOLVED Using the table of standard formation enthalpies that you'll Standard Enthalpy Of Formation C2H2 All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole. Standard Enthalpy Of Formation C2H2.
From oneclass.com
OneClass 2. Calculate the enthalpy of formation of acetylene using Standard Enthalpy Of Formation C2H2 All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1. Standard Enthalpy Of Formation C2H2.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation C2H2 Δh °f δ h ° f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c.. Standard Enthalpy Of Formation C2H2.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Standard Enthalpy Of Formation C2H2 All standard state, 25 °c and 1 bar (written to 1 decimal place). For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: the. Standard Enthalpy Of Formation C2H2.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of acetylene (C2H2 Standard Enthalpy Of Formation C2H2 Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. For example, although oxygen can exist as. Standard Enthalpy Of Formation C2H2.
From slideplayer.com
Unit 10 Energy in Chemical Reactions ppt download Standard Enthalpy Of Formation C2H2 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. All standard state, 25 °c and 1 bar (written to 1 decimal place). Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: standard. Standard Enthalpy Of Formation C2H2.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation C2H2 All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1. Standard Enthalpy Of Formation C2H2.
From www.toppr.com
The molar enthalpies of combustion of C2H2(g) , Cl (graphite) and H2(g Standard Enthalpy Of Formation C2H2 Δh °f δ h ° f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation of any element in its standard state is zero. Standard Enthalpy Of Formation C2H2.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation C2H2 the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. 193 rows in chemistry and thermodynamics, the standard. Standard Enthalpy Of Formation C2H2.
From www.chegg.com
Solved Several reactions and their standard reaction Standard Enthalpy Of Formation C2H2 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. standard enthalpies of formation. the standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation C2H2.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Enthalpy Of Formation C2H2 Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of. Standard Enthalpy Of Formation C2H2.
From www.chegg.com
Calculate the standard Gibbs free energy of reaction Standard Enthalpy Of Formation C2H2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Acetylene is traditionally produced by hydrolysis (reaction with water). Standard Enthalpy Of Formation C2H2.
From www.numerade.com
SOLVED Using the standard formation enthalpies that follow, calculate Standard Enthalpy Of Formation C2H2 Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. standard enthalpies of formation. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most. Standard Enthalpy Of Formation C2H2.
From www.numerade.com
SOLVED Using the standard enthalpies of formation, calculate the Standard Enthalpy Of Formation C2H2 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. All standard state, 25 °c and 1 bar (written to 1 decimal place). the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. Δh °f δ h ° f. Standard Enthalpy Of Formation C2H2.
From www.toppr.com
The molar heats of combustion of C2H2(g), C(graphite) and H2(g) are Standard Enthalpy Of Formation C2H2 All standard state, 25 °c and 1 bar (written to 1 decimal place). Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. standard enthalpies of formation. Δh °f δ h ° f is the enthalpy change for the. Standard Enthalpy Of Formation C2H2.
From www.toppr.com
The molar heats of combustion of C2H2(g), C(graphite) and H2(g) are Standard Enthalpy Of Formation C2H2 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Δh °f δ h. Standard Enthalpy Of Formation C2H2.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation C2H2 All standard state, 25 °c and 1 bar (written to 1 decimal place). the standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide:. Standard Enthalpy Of Formation C2H2.
From www.toppr.com
The molar enthalpies of combustion of C2H2(g) , Cl (graphite) and H2(g Standard Enthalpy Of Formation C2H2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: the standard enthalpy of formation is a measure of the energy. Standard Enthalpy Of Formation C2H2.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation C2H2 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. the standard enthalpy. Standard Enthalpy Of Formation C2H2.
From www.chegg.com
Solved 47, Use the information provided to calculate of the Standard Enthalpy Of Formation C2H2 All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1. Standard Enthalpy Of Formation C2H2.
From www.bartleby.com
Answered Calculate the standard enthalpy of… bartleby Standard Enthalpy Of Formation C2H2 All standard state, 25 °c and 1 bar (written to 1 decimal place). the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Δh °f δ h ° f is. Standard Enthalpy Of Formation C2H2.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation C2H2 standard enthalpies of formation. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Δh °f δ h ° f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy. Standard Enthalpy Of Formation C2H2.
From www.coursehero.com
[Solved] 1. Calculate the standard enthalpy of formation of acetylene Standard Enthalpy Of Formation C2H2 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. standard enthalpies of formation. the standard enthalpy of formation of any element in its standard state is zero by definition. Δh °f δ h ° f is the enthalpy change for the formation of one. Standard Enthalpy Of Formation C2H2.
From www.numerade.com
SOLVED 1. Draw an enthalpy diagram for the reaction between calcium Standard Enthalpy Of Formation C2H2 Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy released. Standard Enthalpy Of Formation C2H2.
From www.chegg.com
Solved Example Enthalpy Changes for a Reaction Calculate 4, Standard Enthalpy Of Formation C2H2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. standard enthalpies of formation. the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ),. Standard Enthalpy Of Formation C2H2.
From www.numerade.com
SOLVED Acetylene, C2H2 (ΔHfo = 234.0 kJ/mol) is sometimes referred to Standard Enthalpy Of Formation C2H2 standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Δh °f δ h ° f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in. 193 rows in chemistry. Standard Enthalpy Of Formation C2H2.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of acetylene (C2H2 Standard Enthalpy Of Formation C2H2 standard enthalpies of formation. All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. the standard enthalpy of formation. Standard Enthalpy Of Formation C2H2.
From exoyndeil.blob.core.windows.net
Standard Enthalpy Of Formation In Elements at Michael Zapien blog Standard Enthalpy Of Formation C2H2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. All standard state, 25 °c and 1 bar (written to 1 decimal place). . Standard Enthalpy Of Formation C2H2.
From www.numerade.com
SOLVED The heat of combustion of acetylene, C2H2(g) + O2(g) → CO2(g Standard Enthalpy Of Formation C2H2 Δh °f δ h ° f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in. the standard enthalpy of formation of any element in its standard state is zero by definition. Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: For example, although oxygen. Standard Enthalpy Of Formation C2H2.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation C2H2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Δh °f δ h ° f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in. the standard enthalpy of formation of any element in its standard state is zero. Standard Enthalpy Of Formation C2H2.
From www.numerade.com
SOLVED QUESTION 1 (15 MARKS) Calculate the standard enthalpy of Standard Enthalpy Of Formation C2H2 the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. standard enthalpies of formation. Acetylene is traditionally produced by hydrolysis (reaction with water) of calcium carbide: For example,. Standard Enthalpy Of Formation C2H2.