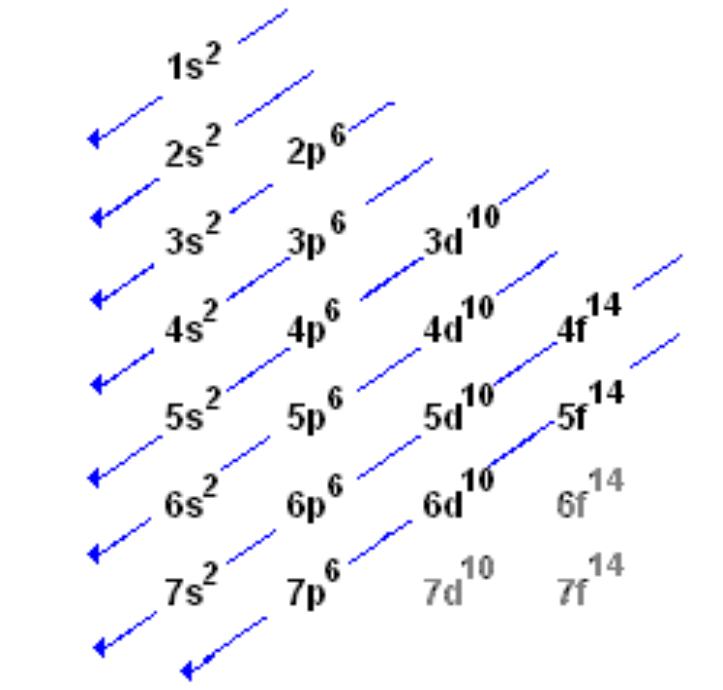
What Kind Of Orbital Does Iron's Electron Configuration End With . Since 1s can only hold two. the initial two electrons in the electron configuration for iron will be in the 1s orbital. Its core orbitals are the. electron configuration chart of all elements is mentioned in the table below. Because the 1s orbital can only hold two electrons, iron’s next two. When we make a 3+ ion for iron, we need to. The shorthand electron configuration (or noble. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as. iron has 26 electrons so its normal electron configuration would be:
from enginedbfascinator.z22.web.core.windows.net
iron has 26 electrons so its normal electron configuration would be: Since 1s can only hold two. in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as. Because the 1s orbital can only hold two electrons, iron’s next two. iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. the initial two electrons in the electron configuration for iron will be in the 1s orbital. electron configuration chart of all elements is mentioned in the table below. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Its core orbitals are the. The shorthand electron configuration (or noble.
Orbital Diagram Notation
What Kind Of Orbital Does Iron's Electron Configuration End With electron configuration chart of all elements is mentioned in the table below. Since 1s can only hold two. The shorthand electron configuration (or noble. electron configuration chart of all elements is mentioned in the table below. iron has 26 electrons so its normal electron configuration would be: in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as. Because the 1s orbital can only hold two electrons, iron’s next two. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. the initial two electrons in the electron configuration for iron will be in the 1s orbital. iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. When we make a 3+ ion for iron, we need to. Its core orbitals are the.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps What Kind Of Orbital Does Iron's Electron Configuration End With the initial two electrons in the electron configuration for iron will be in the 1s orbital. Since 1s can only hold two. When we make a 3+ ion for iron, we need to. iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. electron configuration chart of all. What Kind Of Orbital Does Iron's Electron Configuration End With.
From www.thoughtco.com
Electron Configuration Chart What Kind Of Orbital Does Iron's Electron Configuration End With the initial two electrons in the electron configuration for iron will be in the 1s orbital. iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. Since 1s can only hold two. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. What Kind Of Orbital Does Iron's Electron Configuration End With.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy What Kind Of Orbital Does Iron's Electron Configuration End With Its core orbitals are the. The shorthand electron configuration (or noble. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. the initial two electrons in the electron configuration for iron will. What Kind Of Orbital Does Iron's Electron Configuration End With.
From sciencenotes.org
Iron Atom Science Notes and Projects What Kind Of Orbital Does Iron's Electron Configuration End With When we make a 3+ ion for iron, we need to. the initial two electrons in the electron configuration for iron will be in the 1s orbital. Its core orbitals are the. iron has 26 electrons so its normal electron configuration would be: Because the 1s orbital can only hold two electrons, iron’s next two. in writing. What Kind Of Orbital Does Iron's Electron Configuration End With.
From general.chemistrysteps.com
Electron Configurations Chemistry Steps What Kind Of Orbital Does Iron's Electron Configuration End With iron has 26 electrons so its normal electron configuration would be: in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. Since. What Kind Of Orbital Does Iron's Electron Configuration End With.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps What Kind Of Orbital Does Iron's Electron Configuration End With iron has 26 electrons so its normal electron configuration would be: electron configuration chart of all elements is mentioned in the table below. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. When we make a 3+ ion for iron, we need to. Since 1s can only hold two.. What Kind Of Orbital Does Iron's Electron Configuration End With.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration What Kind Of Orbital Does Iron's Electron Configuration End With Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Because the 1s orbital can only hold two electrons, iron’s next two. in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as. Its core orbitals are. What Kind Of Orbital Does Iron's Electron Configuration End With.
From enginedbtersanctus.z22.web.core.windows.net
Iron Electron Config With Orbital Diagram What Kind Of Orbital Does Iron's Electron Configuration End With Its core orbitals are the. Since 1s can only hold two. When we make a 3+ ion for iron, we need to. iron has 26 electrons so its normal electron configuration would be: in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the. What Kind Of Orbital Does Iron's Electron Configuration End With.
From ar.inspiredpencil.com
Electron Configuration Of Iron What Kind Of Orbital Does Iron's Electron Configuration End With in writing the electron configuration for iron the first two electrons will go in the 1s orbital. electron configuration chart of all elements is mentioned in the table below. iron has 26 electrons so its normal electron configuration would be: Its core orbitals are the. in the case of iron, its electron configuration is written as. What Kind Of Orbital Does Iron's Electron Configuration End With.
From chemistry291.blogspot.com
What Is the Electron Configuration with Step by Step Guides to write What Kind Of Orbital Does Iron's Electron Configuration End With Since 1s can only hold two. Its core orbitals are the. the initial two electrons in the electron configuration for iron will be in the 1s orbital. in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as. electron configuration. What Kind Of Orbital Does Iron's Electron Configuration End With.
From chemsite.lsrhs.net
Electron Configurations What Kind Of Orbital Does Iron's Electron Configuration End With the initial two electrons in the electron configuration for iron will be in the 1s orbital. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. iron has 26 electrons. What Kind Of Orbital Does Iron's Electron Configuration End With.
From ar.inspiredpencil.com
Iron Orbital Notation What Kind Of Orbital Does Iron's Electron Configuration End With iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. Its core orbitals are the. When we make a 3+ ion for iron, we need to. Since 1s can only hold two. The shorthand electron configuration (or noble. the initial two electrons in the electron configuration for iron will. What Kind Of Orbital Does Iron's Electron Configuration End With.
From ar.inspiredpencil.com
Iron Orbital Notation What Kind Of Orbital Does Iron's Electron Configuration End With iron has 26 electrons so its normal electron configuration would be: iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. in the case of iron, its electron configuration. What Kind Of Orbital Does Iron's Electron Configuration End With.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram What Kind Of Orbital Does Iron's Electron Configuration End With iron has 26 electrons so its normal electron configuration would be: in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as. When we make a 3+ ion for iron, we need to. electron configuration chart of all elements is. What Kind Of Orbital Does Iron's Electron Configuration End With.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements What Kind Of Orbital Does Iron's Electron Configuration End With The shorthand electron configuration (or noble. the initial two electrons in the electron configuration for iron will be in the 1s orbital. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. When we make a 3+ ion for iron, we need to. iron is on the fourth row of the periodic table,. What Kind Of Orbital Does Iron's Electron Configuration End With.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts What Kind Of Orbital Does Iron's Electron Configuration End With When we make a 3+ ion for iron, we need to. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as. this. What Kind Of Orbital Does Iron's Electron Configuration End With.
From ar.inspiredpencil.com
Iron Orbital Notation What Kind Of Orbital Does Iron's Electron Configuration End With When we make a 3+ ion for iron, we need to. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. iron has 26 electrons so its normal electron configuration would be: iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. this. What Kind Of Orbital Does Iron's Electron Configuration End With.
From dxoawusbn.blob.core.windows.net
Iron Electron Configuration Long Form at Dennis Amaral blog What Kind Of Orbital Does Iron's Electron Configuration End With in writing the electron configuration for iron the first two electrons will go in the 1s orbital. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. electron configuration chart of all elements is mentioned in the table below. in the case of iron, its electron configuration is. What Kind Of Orbital Does Iron's Electron Configuration End With.
From www.britannica.com
Electronic configuration Definition, Orbitals, & Facts Britannica What Kind Of Orbital Does Iron's Electron Configuration End With iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. electron configuration chart of all elements is mentioned in the table below. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. Its core orbitals are the. Fe 1s 2 2s. What Kind Of Orbital Does Iron's Electron Configuration End With.
From quizlet.com
Draw the electron configuration for a neutral atom of iron. Quizlet What Kind Of Orbital Does Iron's Electron Configuration End With Since 1s can only hold two. When we make a 3+ ion for iron, we need to. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. Its core orbitals are the. electron configuration chart of. What Kind Of Orbital Does Iron's Electron Configuration End With.
From chem.libretexts.org
3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts What Kind Of Orbital Does Iron's Electron Configuration End With iron has 26 electrons so its normal electron configuration would be: in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as. Since 1s can only hold two. The shorthand electron configuration (or noble. the initial two electrons in the. What Kind Of Orbital Does Iron's Electron Configuration End With.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps What Kind Of Orbital Does Iron's Electron Configuration End With When we make a 3+ ion for iron, we need to. Because the 1s orbital can only hold two electrons, iron’s next two. iron has 26 electrons so its normal electron configuration would be: in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of. What Kind Of Orbital Does Iron's Electron Configuration End With.
From ar.inspiredpencil.com
Iron Orbital Notation What Kind Of Orbital Does Iron's Electron Configuration End With Since 1s can only hold two. electron configuration chart of all elements is mentioned in the table below. Its core orbitals are the. iron has 26 electrons so its normal electron configuration would be: in writing the electron configuration for iron the first two electrons will go in the 1s orbital. the initial two electrons in. What Kind Of Orbital Does Iron's Electron Configuration End With.
From ar.inspiredpencil.com
Iron Orbital Notation What Kind Of Orbital Does Iron's Electron Configuration End With the initial two electrons in the electron configuration for iron will be in the 1s orbital. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. The shorthand electron configuration. What Kind Of Orbital Does Iron's Electron Configuration End With.
From www.youtube.com
Iron electronic configuration How to Write Iron electronic What Kind Of Orbital Does Iron's Electron Configuration End With The shorthand electron configuration (or noble. iron has 26 electrons so its normal electron configuration would be: iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. in the. What Kind Of Orbital Does Iron's Electron Configuration End With.
From enginedbfascinator.z22.web.core.windows.net
Orbital Diagram Notation What Kind Of Orbital Does Iron's Electron Configuration End With Since 1s can only hold two. in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as. The shorthand electron configuration (or noble. electron configuration chart of all elements is mentioned in the table below. Fe 1s 2 2s 2 2p. What Kind Of Orbital Does Iron's Electron Configuration End With.
From learninglibrarydyer88.storage.googleapis.com
Electron Configuration And Orbital Notation Worksheet What Kind Of Orbital Does Iron's Electron Configuration End With in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as. Since 1s can only hold two. Because the 1s orbital can only hold two electrons, iron’s next two. The shorthand electron configuration (or noble. the initial two electrons in the. What Kind Of Orbital Does Iron's Electron Configuration End With.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) What Kind Of Orbital Does Iron's Electron Configuration End With electron configuration chart of all elements is mentioned in the table below. in the case of iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as. iron has 26 electrons so its normal electron configuration would be: the initial two electrons in. What Kind Of Orbital Does Iron's Electron Configuration End With.
From manualpentathlon.z14.web.core.windows.net
Electron Configuration And Orbital Diagram What Kind Of Orbital Does Iron's Electron Configuration End With Because the 1s orbital can only hold two electrons, iron’s next two. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble. the initial two electrons in the electron configuration for iron will be in. What Kind Of Orbital Does Iron's Electron Configuration End With.
From chem.libretexts.org
Electronic Configurations Intro Chemistry LibreTexts What Kind Of Orbital Does Iron's Electron Configuration End With Because the 1s orbital can only hold two electrons, iron’s next two. the initial two electrons in the electron configuration for iron will be in the 1s orbital. Since 1s can only hold two. When we make a 3+ ion for iron, we need to. in the case of iron, its electron configuration is written as 1s2 2s2. What Kind Of Orbital Does Iron's Electron Configuration End With.
From www.alamy.com
Diagram of the nuclear composition, electron configuration, and valence What Kind Of Orbital Does Iron's Electron Configuration End With iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. electron configuration chart of all elements is mentioned in the table below. Its core orbitals are the. When we make a 3+ ion for. What Kind Of Orbital Does Iron's Electron Configuration End With.
From mavink.com
Electron Orbital Configuration Chart What Kind Of Orbital Does Iron's Electron Configuration End With Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. iron has 26 electrons so its normal electron configuration would be: in writing the electron configuration for iron the first two electrons will go in the 1s orbital. in the case of iron, its electron configuration is written as 1s2 2s2 2p6. What Kind Of Orbital Does Iron's Electron Configuration End With.
From www.pngegg.com
Free download Electron configuration Atomic orbital Electron shell What Kind Of Orbital Does Iron's Electron Configuration End With Its core orbitals are the. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. in writing the electron configuration for iron the first two electrons will go in the. What Kind Of Orbital Does Iron's Electron Configuration End With.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps What Kind Of Orbital Does Iron's Electron Configuration End With Because the 1s orbital can only hold two electrons, iron’s next two. The shorthand electron configuration (or noble. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. the initial two electrons in the electron configuration for iron will be in the 1s orbital. in the case of iron, its electron configuration is. What Kind Of Orbital Does Iron's Electron Configuration End With.
From byjus.com
Electronic Configuration How To Write Electron ConfigurationChemistry What Kind Of Orbital Does Iron's Electron Configuration End With Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. The shorthand electron configuration (or noble. When we make a 3+ ion for iron, we need to. electron configuration chart of all elements is. What Kind Of Orbital Does Iron's Electron Configuration End With.