Zinc And Hydrochloric Acid Reversible Reaction . The bubbles are hydrogen gas (right side of equation. Zn + cl₂ → zncl₂. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: during this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. zinc reacts with halogens in the presence of moisture: Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. using the zinc and dilute sulfuric acid to make hydrogen in the lab. zinc reacts with hydrochloric acid, hcl (aq), as shown in the following equation. This is a traditional way of producing small amounts of hydrogen in the lab. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. it is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! in this video, we'll be exploring the reaction between zinc metal and. Zn + 2h₂o = zno + h₂↑. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): The reaction is given below.
from www.chegg.com
in this video, we'll be exploring the reaction between zinc metal and. using the zinc and dilute sulfuric acid to make hydrogen in the lab. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. during this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. This is a traditional way of producing small amounts of hydrogen in the lab. zinc reacts with hydrochloric acid, hcl (aq), as shown in the following equation. The reaction is given below. In the process, hydrogen gas is produced. zinc reacts with halogens in the presence of moisture: zinc is oxidized by hydrochloric acid to form zinc chloride.
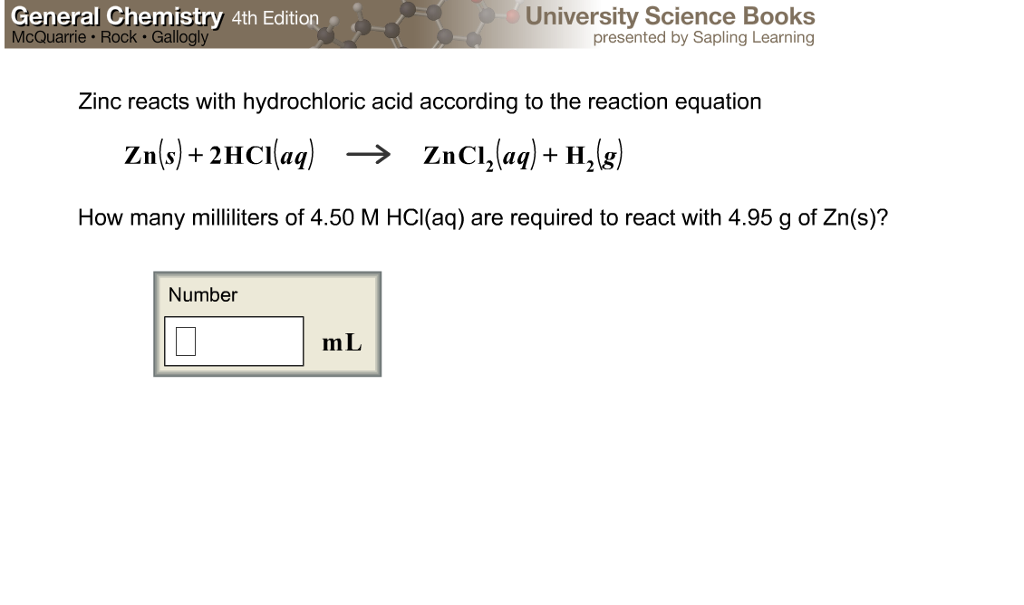
Solved Zinc reacts with hydrochloric acid according to the
Zinc And Hydrochloric Acid Reversible Reaction The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: The bubbles are hydrogen gas (right side of equation. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. zinc reacts with hydrochloric acid, hcl (aq), as shown in the following equation. Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. zinc is oxidized by hydrochloric acid to form zinc chloride. during this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. This is a traditional way of producing small amounts of hydrogen in the lab. zinc reacts with halogens in the presence of moisture: in this video, we'll be exploring the reaction between zinc metal and. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): using the zinc and dilute sulfuric acid to make hydrogen in the lab. it is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! The reaction is given below. Zn + cl₂ → zncl₂.
From www.youtube.com
Zinc and Hydrochloric Acid Reaction chemistry grade10science Zinc And Hydrochloric Acid Reversible Reaction If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: The reaction is given below. In the process, hydrogen gas is produced. zinc is oxidized by hydrochloric acid to form zinc chloride. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus. Zinc And Hydrochloric Acid Reversible Reaction.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science Zinc And Hydrochloric Acid Reversible Reaction zinc is oxidized by hydrochloric acid to form zinc chloride. In the process, hydrogen gas is produced. Zn + cl₂ → zncl₂. it is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. during this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc. Zinc And Hydrochloric Acid Reversible Reaction.
From www.youtube.com
Reaction of Zinc with dilute hydrochloric acid or sulphuric acid....Zn Zinc And Hydrochloric Acid Reversible Reaction The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. In the process, hydrogen gas is produced. The bubbles are hydrogen gas (right side of equation. Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. This is a traditional way of producing small amounts of hydrogen in the lab. Zn + cl₂. Zinc And Hydrochloric Acid Reversible Reaction.
From www.youtube.com
Zinc and 6 M Hydrochloric Acid Reaction YouTube Zinc And Hydrochloric Acid Reversible Reaction zinc reacts with halogens in the presence of moisture: If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: This is a traditional way of producing small amounts of hydrogen in the lab. zinc reacts with hydrochloric acid, hcl (aq), as shown in the following equation. In the process, hydrogen gas is. Zinc And Hydrochloric Acid Reversible Reaction.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0223 Zinc And Hydrochloric Acid Reversible Reaction This is a traditional way of producing small amounts of hydrogen in the lab. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: The bubbles are hydrogen gas (right side of equation. zinc is oxidized by hydrochloric acid to form zinc chloride. Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a. Zinc And Hydrochloric Acid Reversible Reaction.
From askfilo.com
Zinc metal reacts with hydrochloric acid by the following reaction Zn(s).. Zinc And Hydrochloric Acid Reversible Reaction in this video, we'll be exploring the reaction between zinc metal and. Zn + 2h₂o = zno + h₂↑. Zn + cl₂ → zncl₂. it is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! zinc is oxidized by hydrochloric acid to form zinc chloride. This is a traditional way of producing small amounts of hydrogen. Zinc And Hydrochloric Acid Reversible Reaction.
From www.alamy.com
Reaction of zinc with hydrochloric acid Stock Photo Alamy Zinc And Hydrochloric Acid Reversible Reaction when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): The reaction is given below. Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. zinc reacts with halogens in the presence of moisture: it is fairly obvious that zinc metal reacts with aqueous. Zinc And Hydrochloric Acid Reversible Reaction.
From spark.adobe.com
Zinc and Hydrochloric Acid Zinc And Hydrochloric Acid Reversible Reaction zinc is oxidized by hydrochloric acid to form zinc chloride. The reaction is given below. Zn + cl₂ → zncl₂. The bubbles are hydrogen gas (right side of equation. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: This is a traditional way of producing small amounts of hydrogen in the lab.. Zinc And Hydrochloric Acid Reversible Reaction.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0622 Zinc And Hydrochloric Acid Reversible Reaction In the process, hydrogen gas is produced. This is a traditional way of producing small amounts of hydrogen in the lab. using the zinc and dilute sulfuric acid to make hydrogen in the lab. zinc reacts with halogens in the presence of moisture: Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. it is fairly. Zinc And Hydrochloric Acid Reversible Reaction.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc And Hydrochloric Acid Reversible Reaction using the zinc and dilute sulfuric acid to make hydrogen in the lab. The bubbles are hydrogen gas (right side of equation. This is a traditional way of producing small amounts of hydrogen in the lab. zinc is oxidized by hydrochloric acid to form zinc chloride. during this reaction, zinc displaces hydrogen from hydrochloric acid to form. Zinc And Hydrochloric Acid Reversible Reaction.
From www.numerade.com
In this problem, zinc reacts with hydrochloric acid to make zinc Zinc And Hydrochloric Acid Reversible Reaction This is a traditional way of producing small amounts of hydrogen in the lab. it is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure. Zinc And Hydrochloric Acid Reversible Reaction.
From www.youtube.com
Xth Class, Physical Science, Acids, Bases and SaltsReaction of Zinc Zinc And Hydrochloric Acid Reversible Reaction If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: This is a traditional way of producing small amounts of hydrogen in the lab. zinc reacts with halogens in the presence of moisture: using the zinc and dilute sulfuric acid to make hydrogen in the lab. it is fairly obvious that. Zinc And Hydrochloric Acid Reversible Reaction.
From www.numerade.com
SOLVED Consider the reaction between zinc metal and hydrochloric acid Zinc And Hydrochloric Acid Reversible Reaction zinc reacts with hydrochloric acid, hcl (aq), as shown in the following equation. Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. in this video, we'll be exploring the reaction between zinc metal and. Zn + 2h₂o = zno + h₂↑. This is a traditional way of producing small amounts of hydrogen in the lab. The. Zinc And Hydrochloric Acid Reversible Reaction.
From www.youtube.com
Reaction Of Zinc with Hydrochloric acid Chemistry demonstration YouTube Zinc And Hydrochloric Acid Reversible Reaction The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. it is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! using the zinc and dilute sulfuric acid to make hydrogen in the lab. Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. This is a traditional way of. Zinc And Hydrochloric Acid Reversible Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc And Hydrochloric Acid Reversible Reaction This is a traditional way of producing small amounts of hydrogen in the lab. during this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. zinc reacts with hydrochloric acid, hcl (aq), as shown in the following equation. using the zinc and dilute sulfuric acid to make hydrogen in the lab. The. Zinc And Hydrochloric Acid Reversible Reaction.
From www.toppr.com
Zinc and hydrochloric acid react according to the reaction Zn(s Zinc And Hydrochloric Acid Reversible Reaction In the process, hydrogen gas is produced. zinc is oxidized by hydrochloric acid to form zinc chloride. Zn + 2h₂o = zno + h₂↑. during this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: The reaction. Zinc And Hydrochloric Acid Reversible Reaction.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc And Hydrochloric Acid Reversible Reaction Zn + 2h₂o = zno + h₂↑. The bubbles are hydrogen gas (right side of equation. zinc reacts with hydrochloric acid, hcl (aq), as shown in the following equation. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: using the zinc and dilute sulfuric acid to make hydrogen in the lab.. Zinc And Hydrochloric Acid Reversible Reaction.
From byjus.com
Zinc and hydrochloric acid react according t reaction Zn(s) + 2HCI(aq Zinc And Hydrochloric Acid Reversible Reaction using the zinc and dilute sulfuric acid to make hydrogen in the lab. Zn + 2h₂o = zno + h₂↑. zinc reacts with hydrochloric acid, hcl (aq), as shown in the following equation. This is a traditional way of producing small amounts of hydrogen in the lab. during this reaction, zinc displaces hydrogen from hydrochloric acid to. Zinc And Hydrochloric Acid Reversible Reaction.
From www.w3schools.blog
Factors affecting the rate of reaction Concentration W3schools Zinc And Hydrochloric Acid Reversible Reaction Zn + cl₂ → zncl₂. using the zinc and dilute sulfuric acid to make hydrogen in the lab. during this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. in this video, we'll be exploring the reaction between zinc metal and. Zn + 2h₂o = zno + h₂↑. zinc reacts with. Zinc And Hydrochloric Acid Reversible Reaction.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc And Hydrochloric Acid Reversible Reaction This is a traditional way of producing small amounts of hydrogen in the lab. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. in this video, we'll be exploring the reaction between zinc metal and. In the process, hydrogen gas is produced. Zn + 2h₂o = zno + h₂↑. it is. Zinc And Hydrochloric Acid Reversible Reaction.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0309 Science Zinc And Hydrochloric Acid Reversible Reaction Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: The reaction is given below. Zn + 2h₂o = zno + h₂↑. using the zinc and dilute sulfuric acid to make hydrogen in the lab. in this video, we'll be exploring. Zinc And Hydrochloric Acid Reversible Reaction.
From www.youtube.com
Chemical Reaction zinc and hydrochloric acid YouTube Zinc And Hydrochloric Acid Reversible Reaction Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: In the process, hydrogen gas is produced. zinc reacts. Zinc And Hydrochloric Acid Reversible Reaction.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc And Hydrochloric Acid Reversible Reaction using the zinc and dilute sulfuric acid to make hydrogen in the lab. This is a traditional way of producing small amounts of hydrogen in the lab. The bubbles are hydrogen gas (right side of equation. Zn + 2h₂o = zno + h₂↑. Zn + cl₂ → zncl₂. In the process, hydrogen gas is produced. The reaction is given. Zinc And Hydrochloric Acid Reversible Reaction.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid Reversible Reaction it is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! in this video, we'll be exploring the reaction between zinc metal and. zinc reacts with hydrochloric acid, hcl (aq), as shown in the following equation. zinc reacts with halogens in the presence of moisture: In the process, hydrogen gas is produced. This is a. Zinc And Hydrochloric Acid Reversible Reaction.
From slideplayer.com
Chemical Reactions & Equations ppt download Zinc And Hydrochloric Acid Reversible Reaction zinc is oxidized by hydrochloric acid to form zinc chloride. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. zinc reacts with halogens in the presence of moisture: it is fairly obvious. Zinc And Hydrochloric Acid Reversible Reaction.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0662 Zinc And Hydrochloric Acid Reversible Reaction zinc reacts with halogens in the presence of moisture: In the process, hydrogen gas is produced. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): in this video, we'll be exploring the reaction between zinc metal and. If a piece of zinc is immersed. Zinc And Hydrochloric Acid Reversible Reaction.
From jaylongokelawrence.blogspot.com
Zinc Reacts With Hydrochloric Acid Zinc And Hydrochloric Acid Reversible Reaction This is a traditional way of producing small amounts of hydrogen in the lab. zinc reacts with hydrochloric acid, hcl (aq), as shown in the following equation. Zn + cl₂ → zncl₂. during this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. in this video, we'll be exploring the reaction between. Zinc And Hydrochloric Acid Reversible Reaction.
From askfilo.com
13. Zinc and hydrochloric acid react according to the reaction Zn(s)+2HCl.. Zinc And Hydrochloric Acid Reversible Reaction using the zinc and dilute sulfuric acid to make hydrogen in the lab. in this video, we'll be exploring the reaction between zinc metal and. zinc is oxidized by hydrochloric acid to form zinc chloride. The bubbles are hydrogen gas (right side of equation. it is fairly obvious that zinc metal reacts with aqueous hydrochloric acid!. Zinc And Hydrochloric Acid Reversible Reaction.
From dxorwmpbw.blob.core.windows.net
Word Equation Between Zinc And Hydrochloric Acid at Noah Brooks blog Zinc And Hydrochloric Acid Reversible Reaction If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: In the process, hydrogen gas is produced. Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. This is a traditional way of producing small amounts of hydrogen in the lab. in this video, we'll be exploring the reaction between zinc metal. Zinc And Hydrochloric Acid Reversible Reaction.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Zinc And Hydrochloric Acid Reversible Reaction zinc reacts with halogens in the presence of moisture: The reaction is given below. In the process, hydrogen gas is produced. Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. zinc reacts with hydrochloric acid, hcl (aq), as shown in the following equation. Zn + cl₂ → zncl₂. The bubbles are hydrogen gas (right side of. Zinc And Hydrochloric Acid Reversible Reaction.
From dxopfgxpq.blob.core.windows.net
Zinc And Hydrochloric Acid Reaction Explained at John Crawford blog Zinc And Hydrochloric Acid Reversible Reaction The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. This is a traditional way of producing small amounts of hydrogen in the lab. it is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: In. Zinc And Hydrochloric Acid Reversible Reaction.
From thewonderofscience.com
Reaction of zinc and hydrochloric acid (NY) — The Wonder of Science Zinc And Hydrochloric Acid Reversible Reaction during this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. it is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! in this video, we'll be exploring the reaction between zinc metal and. Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. If a piece of zinc is. Zinc And Hydrochloric Acid Reversible Reaction.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction Zinc And Hydrochloric Acid Reversible Reaction The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. during this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. zinc reacts with hydrochloric acid, hcl (aq), as shown in the following equation. This is a traditional way of producing small amounts of hydrogen in the. Zinc And Hydrochloric Acid Reversible Reaction.
From melscience.com
The reaction between hydrochloric acid and zinc MEL Chemistry Zinc And Hydrochloric Acid Reversible Reaction it is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! Zn + 2h₂o = zno + h₂↑. Zn(s) + 2hcl (aq) → zncl 2(aq) + h2(g) a student. This is a traditional way of producing small amounts of hydrogen in the lab. The bubbles are hydrogen gas (right side of equation. using the zinc and dilute. Zinc And Hydrochloric Acid Reversible Reaction.
From warreninstitute.org
Unlock The POWER Of Chemical Reaction Zn + HCl To Zinc + Hydrochloric Acid Zinc And Hydrochloric Acid Reversible Reaction in this video, we'll be exploring the reaction between zinc metal and. using the zinc and dilute sulfuric acid to make hydrogen in the lab. zinc is oxidized by hydrochloric acid to form zinc chloride. The reaction is given below. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. Zn. Zinc And Hydrochloric Acid Reversible Reaction.