Biological Buffers Function To Achieve A Ph Range Of . The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Buffers are important in biological systems because of their. Buffers are essential to life. They help maintain the proper functioning of cellular systems by resisting rapid. Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. Biological buffers are compounds that help the body maintain a ph around 7.4. How to prepare biological buffers? Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess hydrogen ions, thereby maintaining the. Maintenance of biological ph is important.
from www.youtube.com
The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. Buffers are important in biological systems because of their. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess hydrogen ions, thereby maintaining the. Biological buffers are compounds that help the body maintain a ph around 7.4. Maintenance of biological ph is important. They help maintain the proper functioning of cellular systems by resisting rapid. How to prepare biological buffers?
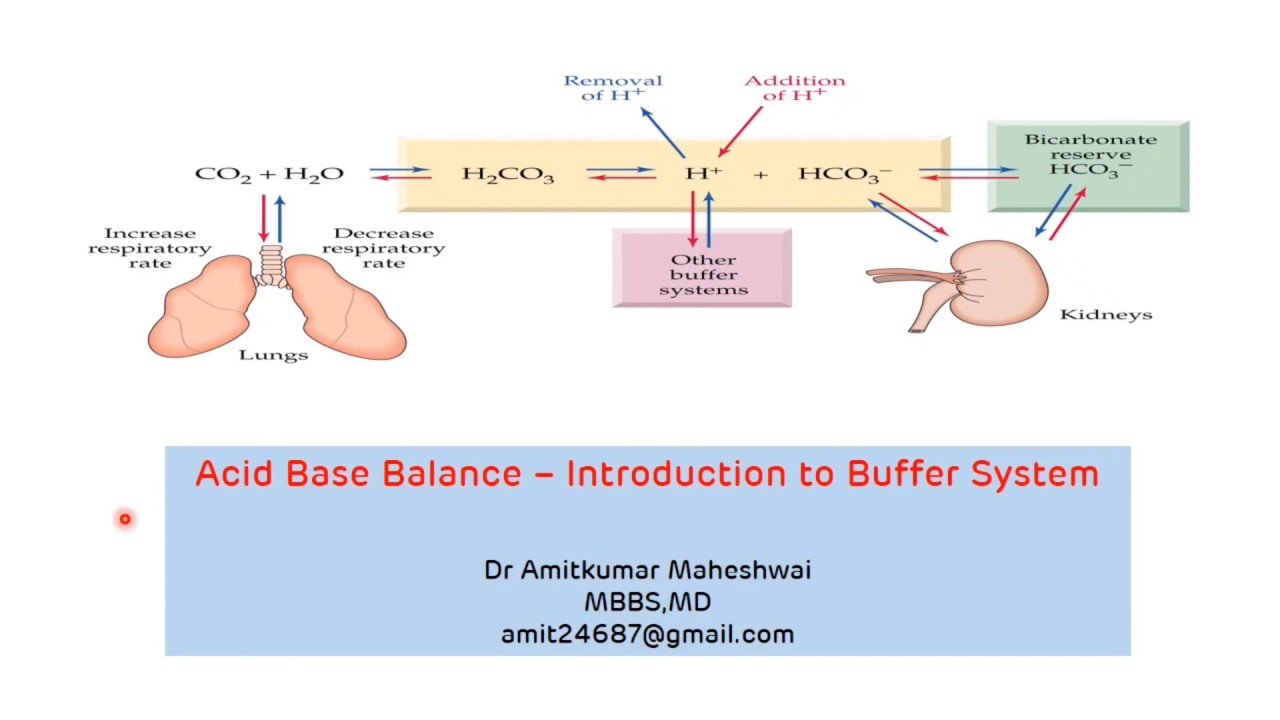
Introduction to Buffer System Regulation of pH Acid Base Balance
Biological Buffers Function To Achieve A Ph Range Of Buffers are essential to life. They help maintain the proper functioning of cellular systems by resisting rapid. Biological buffers are organic substances that help regulate the ph level in organisms. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the. Biological buffers are compounds that help the body maintain a ph around 7.4. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. Buffers are important in biological systems because of their. Maintenance of biological ph is important. Buffers are essential to life. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. How to prepare biological buffers?
From slideplayer.com
Bonds in Biology Weak bonds Strong bonds Hydrogen bond hydrogen bonds Biological Buffers Function To Achieve A Ph Range Of They act by neutralizing excess hydrogen ions, thereby maintaining the. Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. How to prepare biological buffers? The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are compounds that help the body maintain. Biological Buffers Function To Achieve A Ph Range Of.
From www.reagent.co.uk
How Do Biological Buffers Work? The Science Blog Biological Buffers Function To Achieve A Ph Range Of Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. Maintenance of biological ph is important. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are important in biological systems because of their. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Buffers Function To Achieve A Ph Range Of Biological buffers are compounds that help the body maintain a ph around 7.4. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. They act by neutralizing excess hydrogen ions, thereby maintaining the.. Biological Buffers Function To Achieve A Ph Range Of.
From www.learner.org
The pH Scale (animation) Annenberg Learner Biological Buffers Function To Achieve A Ph Range Of The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. How to prepare biological buffers? Biological buffers are. Biological Buffers Function To Achieve A Ph Range Of.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Biological Buffers Function To Achieve A Ph Range Of Buffers are essential to life. Biological buffers are organic substances that help regulate the ph level in organisms. Maintenance of biological ph is important. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. The buffer systems functioning in blood plasma include. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Biological Buffers Function To Achieve A Ph Range Of The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. They help maintain the proper functioning of cellular systems by resisting rapid. Biological buffers are organic substances that. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Buffers Function To Achieve A Ph Range Of Biological buffers are compounds that help the body maintain a ph around 7.4. Buffers are important in biological systems because of their. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. How to prepare biological buffers? The buffer systems functioning in blood plasma include plasma. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideshare.net
Buffers in biological systems Biological Buffers Function To Achieve A Ph Range Of The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Biological buffers are organic substances that help regulate the ph level in organisms. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. They help maintain the proper. Biological Buffers Function To Achieve A Ph Range Of.
From webmis.highland.cc.il.us
Buffers Biological Buffers Function To Achieve A Ph Range Of How to prepare biological buffers? The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the. Biological buffers are compounds that help the body maintain a ph around 7.4. Buffers are solutions that moderate ph changes when an acid or base is added to. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Buffers Function To Achieve A Ph Range Of Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. They help maintain the proper functioning of cellular systems by resisting rapid. Buffers are important in biological systems because of their. Buffers are essential. Biological Buffers Function To Achieve A Ph Range Of.
From www.hopaxfc.com
Biological Buffers Products Hopax Fine Chemicals Biological Buffers Function To Achieve A Ph Range Of The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Maintenance of biological ph is important. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. They help maintain the proper functioning of cellular systems by resisting rapid.. Biological Buffers Function To Achieve A Ph Range Of.
From joifezsdx.blob.core.windows.net
Biological Buffers Ppt at Erin Dodds blog Biological Buffers Function To Achieve A Ph Range Of The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Buffers are important in biological systems because of their. They act by neutralizing excess hydrogen ions, thereby maintaining. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideserve.com
PPT Cell Biology Cell Compounds and Biological Molecules PowerPoint Biological Buffers Function To Achieve A Ph Range Of They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. They help maintain the proper functioning of cellular systems by resisting rapid. How to prepare biological buffers? Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are. Biological Buffers Function To Achieve A Ph Range Of.
From docslib.org
Biological Buffers Reference Chart DocsLib Biological Buffers Function To Achieve A Ph Range Of Biological buffers are organic substances that help regulate the ph level in organisms. How to prepare biological buffers? The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers are important in biological systems because of their. They act by neutralizing excess hydrogen ions, thereby maintaining the. The purpose of a buffer in. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Biological Buffers Function To Achieve A Ph Range Of Buffers are essential to life. They act by neutralizing excess hydrogen ions, thereby maintaining the. Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers are solutions that moderate ph changes when an. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 Biological Buffers Function To Achieve A Ph Range Of They help maintain the proper functioning of cellular systems by resisting rapid. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are essential to life. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. How to prepare biological buffers? Maintenance of biological ph is important. Biological buffers are organic. Biological Buffers Function To Achieve A Ph Range Of.
From teknova.com
Antifoam Teknova Teknova Biological Buffers Function To Achieve A Ph Range Of They help maintain the proper functioning of cellular systems by resisting rapid. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Buffers are important in biological systems because. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 Biological Buffers Function To Achieve A Ph Range Of How to prepare biological buffers? They help maintain the proper functioning of cellular systems by resisting rapid. Biological buffers are compounds that help the body maintain a ph around 7.4. Maintenance of biological ph is important. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are organic substances that help. Biological Buffers Function To Achieve A Ph Range Of.
From studylib.net
Good's buffers (biological buffers) Biological Buffers Function To Achieve A Ph Range Of They help maintain the proper functioning of cellular systems by resisting rapid. Buffers are important in biological systems because of their. Biological buffers are compounds that help the body maintain a ph around 7.4. Buffers are essential to life. They act by neutralizing excess hydrogen ions, thereby maintaining the. Biological buffers are organic substances that help regulate the ph level. Biological Buffers Function To Achieve A Ph Range Of.
From derangedphysiology.com
Buffers and buffering power Deranged Physiology Biological Buffers Function To Achieve A Ph Range Of How to prepare biological buffers? Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Buffers are important in biological systems because of their. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Maintenance of biological ph. Biological Buffers Function To Achieve A Ph Range Of.
From www.researchgate.net
Examples of buffer compounds divided into accepted physiological Biological Buffers Function To Achieve A Ph Range Of How to prepare biological buffers? Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. They help maintain the proper functioning of cellular systems by resisting rapid. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. The. Biological Buffers Function To Achieve A Ph Range Of.
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog Biological Buffers Function To Achieve A Ph Range Of Biological buffers are compounds that help the body maintain a ph around 7.4. Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. How to prepare biological buffers? Maintenance of biological ph is important. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are essential to life. They help. Biological Buffers Function To Achieve A Ph Range Of.
From www.reagent.co.uk
How Do Biological Buffers Work? The Science Blog Biological Buffers Function To Achieve A Ph Range Of Biological buffers are compounds that help the body maintain a ph around 7.4. Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are important in biological systems because of their. The purpose of a buffer in a biological system. Biological Buffers Function To Achieve A Ph Range Of.
From slideplayer.com
Water Properties & pH Biology I. ppt download Biological Buffers Function To Achieve A Ph Range Of Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. They help maintain the proper functioning of cellular systems by resisting rapid. They act by neutralizing excess hydrogen ions, thereby maintaining the. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers are important. Biological Buffers Function To Achieve A Ph Range Of.
From slideplayer.com
Introduction to Biochemistry ppt download Biological Buffers Function To Achieve A Ph Range Of They act by neutralizing excess hydrogen ions, thereby maintaining the. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. They help maintain the proper functioning of cellular systems by resisting rapid. Biological buffers are organic substances that help regulate the ph level in organisms. Buffers are solutions that moderate ph changes when. Biological Buffers Function To Achieve A Ph Range Of.
From www.youtube.com
pH and buffers overview for biology YouTube Biological Buffers Function To Achieve A Ph Range Of Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. How to prepare biological buffers? They help maintain the proper functioning of cellular systems by resisting rapid. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biological buffers are compounds that help. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideserve.com
PPT Novel Aminomethanesulfonate Buffers for Biological Buffering in Biological Buffers Function To Achieve A Ph Range Of The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and.. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 Biological Buffers Function To Achieve A Ph Range Of The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Maintenance of biological ph is important. How to prepare biological buffers? They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are essential to life. Buffers are solutions that moderate ph changes when an acid. Biological Buffers Function To Achieve A Ph Range Of.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Biological Buffers Function To Achieve A Ph Range Of Biological buffers are organic substances that help regulate the ph level in organisms. Maintenance of biological ph is important. They help maintain the proper functioning of cellular systems by resisting rapid. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Lower ph values are associated. Biological Buffers Function To Achieve A Ph Range Of.
From slideplayer.com
Biology Concepts & Applications ppt download Biological Buffers Function To Achieve A Ph Range Of How to prepare biological buffers? Biological buffers are compounds that help the body maintain a ph around 7.4. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. Biological buffers are organic substances that. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideserve.com
PPT Novel Aminomethanesulfonate Buffers for Biological Buffering in Biological Buffers Function To Achieve A Ph Range Of Biological buffers are compounds that help the body maintain a ph around 7.4. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biological buffers are organic substances that help regulate the ph level in organisms. Buffers are essential to life. They help maintain the proper functioning of cellular systems by resisting. Biological Buffers Function To Achieve A Ph Range Of.
From www.osmosis.org
Physiologic pH and buffers Video, Anatomy & Definition Osmosis Biological Buffers Function To Achieve A Ph Range Of The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. How to prepare biological buffers? Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate. Biological Buffers Function To Achieve A Ph Range Of.
From www.slideserve.com
PPT THEME Acidbase equilibrium in biological systems. Buffer Biological Buffers Function To Achieve A Ph Range Of They help maintain the proper functioning of cellular systems by resisting rapid. They act by neutralizing excess hydrogen ions, thereby maintaining the. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers are important in biological systems because of their. Biological buffers are organic substances that help regulate the ph level in. Biological Buffers Function To Achieve A Ph Range Of.
From www.semanticscholar.org
[PDF] (Un)suitability of the use of pH buffers in biological Biological Buffers Function To Achieve A Ph Range Of Biological buffers are compounds that help the body maintain a ph around 7.4. Buffers are essential to life. Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. Biological Buffers Function To Achieve A Ph Range Of.
From www.youtube.com
Buffers and pH Biology YouTube Biological Buffers Function To Achieve A Ph Range Of Lower ph values are associated with metabolic and respiratory acidosis while higher ph values are characteristic of metabolic and. They help maintain the proper functioning of cellular systems by resisting rapid. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biological buffers are compounds that help the body maintain a ph. Biological Buffers Function To Achieve A Ph Range Of.