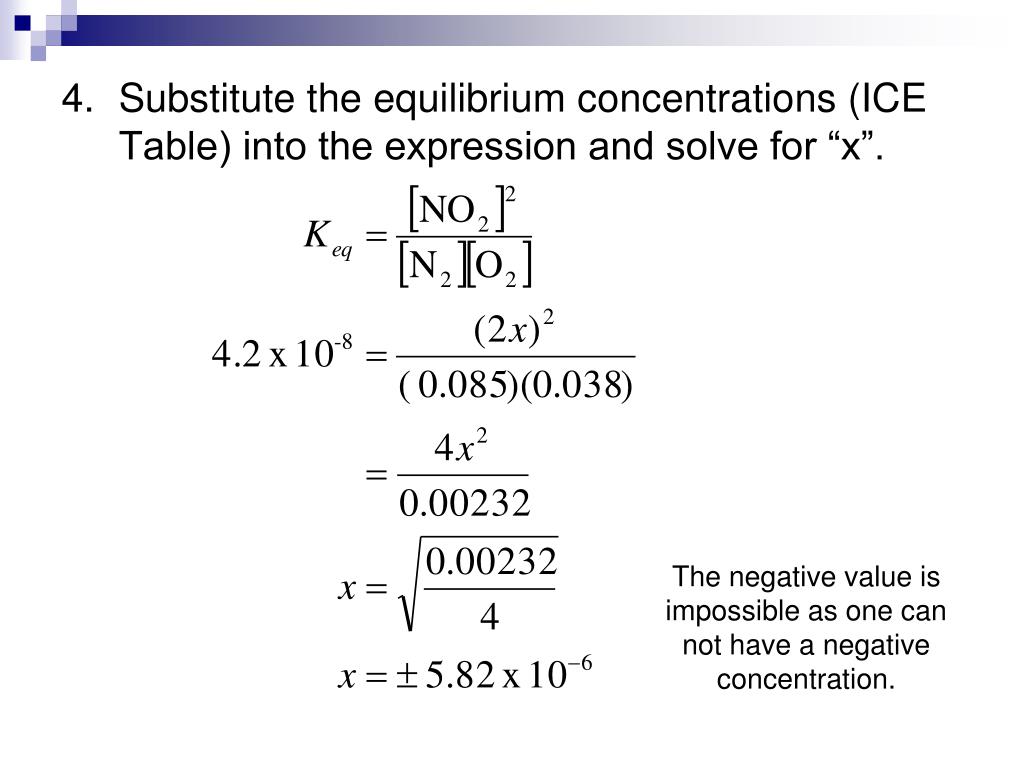
Ice Table Equilibrium Concentration . an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. Ice tables also help to solve many other types of equilibrium problems. write the equilibrium equation for the reaction. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. Follow the steps to write the. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. learn how to use ice tables to calculate equilibrium constants for reversible reactions. Substitute appropriate values from the ice table to obtain \(x\). Ice tables are simple matrices. An ice chart shows the initial, change and equilibrium.
from www.slideserve.com
Ice tables are simple matrices. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. An ice chart shows the initial, change and equilibrium. learn how to use ice tables to calculate equilibrium constants for reversible reactions. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. Substitute appropriate values from the ice table to obtain \(x\). Follow the steps to write the. write the equilibrium equation for the reaction. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction.
PPT Quantitative Changes in Equilibrium Systems PowerPoint Presentation ID2740669
Ice Table Equilibrium Concentration learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. Ice tables are simple matrices. write the equilibrium equation for the reaction. Ice tables also help to solve many other types of equilibrium problems. An ice chart shows the initial, change and equilibrium. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. Follow the steps to write the. Substitute appropriate values from the ice table to obtain \(x\). an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. learn how to use ice tables to calculate equilibrium constants for reversible reactions.
From www.youtube.com
Equilibrium 5.4 ICE Tables YouTube Ice Table Equilibrium Concentration learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. Follow the steps to write the. Substitute appropriate values from the ice table to obtain \(x\). Ice tables are simple matrices. learn how to use ice. Ice Table Equilibrium Concentration.
From www.slideserve.com
PPT Calculating Equilibrium Concentrations from Initial Concentrations PowerPoint Presentation Ice Table Equilibrium Concentration learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. Follow the steps to write the. Ice tables also help to solve many other types of equilibrium problems. learn how to use ice tables to calculate equilibrium constants for reversible reactions. learn how to use ice tables (initial,. Ice Table Equilibrium Concentration.
From www.studocu.com
Determining K, Equilibrium Concentration Using ICE Tables Q vs. K Determining K, Equilibrium Ice Table Equilibrium Concentration learn how to use ice tables to calculate equilibrium constants for reversible reactions. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. write the equilibrium. Ice Table Equilibrium Concentration.
From www.slideserve.com
PPT Quantitative Changes in Equilibrium Systems PowerPoint Presentation ID2740669 Ice Table Equilibrium Concentration Ice tables are simple matrices. learn how to use ice tables to calculate equilibrium constants for reversible reactions. Ice tables also help to solve many other types of equilibrium problems. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. Substitute appropriate values from the ice table to obtain \(x\). An ice. Ice Table Equilibrium Concentration.
From www.youtube.com
How to find equilibrium concentrations using an ICE table YouTube Ice Table Equilibrium Concentration Ice tables are simple matrices. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. Substitute appropriate values from the ice table to obtain \(x\). Ice tables also help to solve many other types of equilibrium problems. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry.. Ice Table Equilibrium Concentration.
From www.youtube.com
How To Calculate Equilibrium Concentrations Using ICE Tables! YouTube Ice Table Equilibrium Concentration Follow the steps to write the. Ice tables also help to solve many other types of equilibrium problems. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. write the equilibrium equation. Ice Table Equilibrium Concentration.
From www.slideserve.com
PPT ICE Tables PowerPoint Presentation, free download ID2031538 Ice Table Equilibrium Concentration an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. Ice tables are simple matrices. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. learn how to make an ice chart, a useful tool for solving equilibrium. Ice Table Equilibrium Concentration.
From www.youtube.com
InitialChangeEquilibrium (ICE) Tables YouTube Ice Table Equilibrium Concentration learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. learn how to use ice tables to calculate equilibrium constants for reversible reactions. Substitute appropriate values from the ice table to obtain \(x\). write the. Ice Table Equilibrium Concentration.
From www.numerade.com
SOLVED Setting up an ICE table to calculate Kc Iodine molecules react reversibly with iodide Ice Table Equilibrium Concentration learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. an ice table (for initial concentration,. Ice Table Equilibrium Concentration.
From slidetodoc.com
ICE Tables and Equilibrium Concentrations Lesson Outline ICE Ice Table Equilibrium Concentration learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. Ice tables are simple matrices. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant. Ice Table Equilibrium Concentration.
From www.youtube.com
Equilibrium Calculations ICE Table w/ Equilibrium Concentration Given YouTube Ice Table Equilibrium Concentration Ice tables are simple matrices. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. An ice chart shows the initial, change and equilibrium. Follow the steps to write the. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an. Ice Table Equilibrium Concentration.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID3098443 Ice Table Equilibrium Concentration Ice tables are simple matrices. learn how to use ice tables to calculate equilibrium constants for reversible reactions. An ice chart shows the initial, change and equilibrium. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. Substitute appropriate values from the ice table. Ice Table Equilibrium Concentration.
From slidetodoc.com
Chemical Equilibrium What is equilibrium Expressions for equilibrium Ice Table Equilibrium Concentration An ice chart shows the initial, change and equilibrium. Substitute appropriate values from the ice table to obtain \(x\). Follow the steps to write the. write the equilibrium equation for the reaction. Ice tables also help to solve many other types of equilibrium problems. learn how to use ice tables to find the equilibrium concentrations of reactants and. Ice Table Equilibrium Concentration.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube Ice Table Equilibrium Concentration Ice tables are simple matrices. Substitute appropriate values from the ice table to obtain \(x\). write the equilibrium equation for the reaction. learn how to use ice tables to calculate equilibrium constants for reversible reactions. An ice chart shows the initial, change and equilibrium. learn how to use ice tables to find the equilibrium concentrations of reactants. Ice Table Equilibrium Concentration.
From www.youtube.com
Chemical Equilibrium Ice Table Equilibrium Constant Expression, Initial Concentration, Kp Ice Table Equilibrium Concentration write the equilibrium equation for the reaction. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. learn how to make an ice. Ice Table Equilibrium Concentration.
From www.conquerhsc.com
HSC Chemistry Module 5 Inquiry Question 3 Ice Table Equilibrium Concentration Substitute appropriate values from the ice table to obtain \(x\). write the equilibrium equation for the reaction. Ice tables are simple matrices. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. learn how to make an ice chart, a useful tool for. Ice Table Equilibrium Concentration.
From www.chegg.com
Solved ICE tables are used for calculating changes in Ice Table Equilibrium Concentration Ice tables are simple matrices. learn how to use ice tables to calculate equilibrium constants for reversible reactions. An ice chart shows the initial, change and equilibrium. Ice tables also help to solve many other types of equilibrium problems. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an. Ice Table Equilibrium Concentration.
From studylib.net
How to calculate equilibrium concentrations using ICE table Ice Table Equilibrium Concentration write the equilibrium equation for the reaction. learn how to use ice tables to calculate equilibrium constants for reversible reactions. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. Substitute appropriate values from the ice table to obtain \(x\). Follow the steps to write the. Ice tables. Ice Table Equilibrium Concentration.
From www.youtube.com
Solving Equilibrium ICE Tables WITHOUT the Quadratic Formula YouTube Ice Table Equilibrium Concentration Follow the steps to write the. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. learn how to use ice tables to calculate equilibrium constants for reversible reactions. Ice tables also help to solve many other types of equilibrium problems. Ice tables are simple matrices. learn how. Ice Table Equilibrium Concentration.
From www.chegg.com
Solved We use an ICE table to solve for equilibrium Ice Table Equilibrium Concentration write the equilibrium equation for the reaction. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. Substitute appropriate values from the ice table to obtain \(x\). An ice chart shows the initial, change and equilibrium. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium. Ice Table Equilibrium Concentration.
From slidetodoc.com
ICE Tables and Equilibrium Concentrations Lesson Outline ICE Ice Table Equilibrium Concentration Substitute appropriate values from the ice table to obtain \(x\). learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. Ice tables are simple matrices. an ice table (for initial concentration, change in concentration, and equilibrium. Ice Table Equilibrium Concentration.
From www.youtube.com
Chemical Equilibrium Ice Table Equilibrium Constant Expression, Initial Concentration, Kp Ice Table Equilibrium Concentration Follow the steps to write the. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. Substitute appropriate values from the ice table to obtain \(x\). write the equilibrium equation for the reaction.. Ice Table Equilibrium Concentration.
From www.chegg.com
Solved 3) ICE tables equilibrium concentration/mass 1/2 Ice Table Equilibrium Concentration learn how to use ice tables to calculate equilibrium constants for reversible reactions. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. Follow the steps to write the. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. Ice tables are simple. Ice Table Equilibrium Concentration.
From slidetodoc.com
ICE Tables and Equilibrium Concentrations Lesson Outline ICE Ice Table Equilibrium Concentration Ice tables are simple matrices. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. learn how to use ice tables to calculate equilibrium constants for reversible reactions. An ice chart shows the initial, change and equilibrium. Substitute appropriate values from the ice table to obtain \(x\). write. Ice Table Equilibrium Concentration.
From www.youtube.com
Calculate Equilibrium [ICE Tables] YouTube Ice Table Equilibrium Concentration learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. Ice tables are simple matrices. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. write. Ice Table Equilibrium Concentration.
From www.youtube.com
Chemical Equilibrium Ice Table Equilibrium Constant Expression, Initial Concentration, Kp Ice Table Equilibrium Concentration learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. Ice tables are simple matrices. Ice tables also help to solve many other types of equilibrium problems. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. . Ice Table Equilibrium Concentration.
From www.studocu.com
Outline 2 Equilibrium Concentrations using ICE Tables, Reaction Quotient, Predicting Direction Ice Table Equilibrium Concentration learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. Substitute appropriate values from the ice table to obtain \(x\). learn how to use ice tables to calculate equilibrium constants for reversible reactions. write the equilibrium equation for the reaction. an ice table (for initial concentration, change in concentration, and. Ice Table Equilibrium Concentration.
From www.chegg.com
Solved Predict the equilibrium concentration of Br in the Ice Table Equilibrium Concentration an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. An ice chart shows the initial, change and equilibrium. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. Ice tables also help to solve many other types. Ice Table Equilibrium Concentration.
From www.youtube.com
ICE Tables Given K but NO Equilibrium Concentrations YouTube Ice Table Equilibrium Concentration An ice chart shows the initial, change and equilibrium. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. Substitute appropriate values from the ice table to obtain \(x\). Follow the steps to write the. Ice tables are simple matrices. learn how to make an ice chart, a useful. Ice Table Equilibrium Concentration.
From www.youtube.com
Quadratic Equation ICE Table Equilibrium Calculations YouTube Ice Table Equilibrium Concentration an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. Substitute appropriate values from the ice table to obtain \(x\). Follow the steps to write the. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. learn how. Ice Table Equilibrium Concentration.
From www.youtube.com
Perfect Squares ICE Table Equilibrium Calculations YouTube Ice Table Equilibrium Concentration An ice chart shows the initial, change and equilibrium. Ice tables also help to solve many other types of equilibrium problems. write the equilibrium equation for the reaction. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. Ice tables are simple matrices. Follow. Ice Table Equilibrium Concentration.
From www.chegg.com
Solved 3) ICE tables equilibrium concentration/mass Ice Table Equilibrium Concentration Follow the steps to write the. Substitute appropriate values from the ice table to obtain \(x\). Ice tables are simple matrices. learn how to use ice tables to find the equilibrium concentrations of reactants and products in a chemical reaction. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. write the. Ice Table Equilibrium Concentration.
From www.youtube.com
ICE Tables Solving for Equilibrium Concentration YouTube Ice Table Equilibrium Concentration An ice chart shows the initial, change and equilibrium. learn how to use ice tables to calculate equilibrium constants for reversible reactions. Ice tables are simple matrices. learn how to make an ice chart, a useful tool for solving equilibrium problems in chemistry. write the equilibrium equation for the reaction. an ice table (for initial concentration,. Ice Table Equilibrium Concentration.
From trenahaziim.blogspot.com
13+ Equilibrium Calculations With Ice Tables Worksheet TrenaHaziim Ice Table Equilibrium Concentration Ice tables are simple matrices. An ice chart shows the initial, change and equilibrium. Follow the steps to write the. Ice tables also help to solve many other types of equilibrium problems. Substitute appropriate values from the ice table to obtain \(x\). learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in chemistry. learn. Ice Table Equilibrium Concentration.
From www.youtube.com
Reaction quotients and ICE tables YouTube Ice Table Equilibrium Concentration Substitute appropriate values from the ice table to obtain \(x\). Follow the steps to write the. Ice tables also help to solve many other types of equilibrium problems. write the equilibrium equation for the reaction. An ice chart shows the initial, change and equilibrium. learn how to use ice tables (initial, change, equilibrium) to solve equilibrium problems in. Ice Table Equilibrium Concentration.