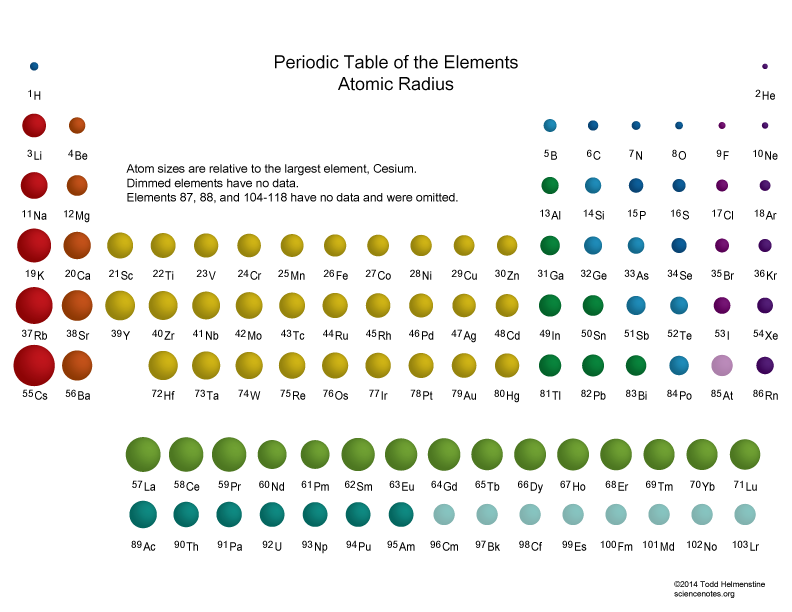
Explain Shielding And How It Changes On The Periodic Table . Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. Electrons in an \ (s\) orbital can shield \ (p\). The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. Moving across a period, z_eff. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in.
from periodictrendsforhonorschemistry.weebly.com
The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. Moving across a period, z_eff. The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in. Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. Electrons in an \ (s\) orbital can shield \ (p\).
Atomic Radius Trends of the Periodic Table
Explain Shielding And How It Changes On The Periodic Table Electrons in an \ (s\) orbital can shield \ (p\). Moving across a period, z_eff. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. The net positive charge from the nucleus that an electron can “feel” attractions from. Electrons in an \ (s\) orbital can shield \ (p\). Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in.
From sites.google.com
The Periodic Table Final Review Assignment Explain Shielding And How It Changes On The Periodic Table The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. The net positive charge from the nucleus that an electron can “feel” attractions from. Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The shielding effect, also known as the screening effect, is. Explain Shielding And How It Changes On The Periodic Table.
From fyoyhsxem.blob.core.windows.net
What Is Shielding In An Atom at Leslie Fields blog Explain Shielding And How It Changes On The Periodic Table Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. Moving across a period, z_eff. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The shielding effect, also known as the screening effect, is the decrease in the. Explain Shielding And How It Changes On The Periodic Table.
From www.slideshare.net
Periodic table Explain Shielding And How It Changes On The Periodic Table The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. Electrons in an \ (s\) orbital can shield \ (p\). The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. The net positive charge from the nucleus that an. Explain Shielding And How It Changes On The Periodic Table.
From www.youtube.com
periodic table nuclear charge and shielding YouTube Explain Shielding And How It Changes On The Periodic Table Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence. Explain Shielding And How It Changes On The Periodic Table.
From www.chemistrylearner.com
Periodic Trends Definition and Properties Explain Shielding And How It Changes On The Periodic Table The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in. The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in. Explain Shielding And How It Changes On The Periodic Table.
From perso.numericable.fr
Atomic and ionic radius increases due to increased electron shielding Explain Shielding And How It Changes On The Periodic Table Electrons in an \ (s\) orbital can shield \ (p\). Moving across a period, z_eff. Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect increases with the number of electrons between the nucleus and. Explain Shielding And How It Changes On The Periodic Table.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision Explain Shielding And How It Changes On The Periodic Table The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in. Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded. Explain Shielding And How It Changes On The Periodic Table.
From www.numerade.com
What is shielding? Match the items in the left column to the Explain Shielding And How It Changes On The Periodic Table Electrons in an \ (s\) orbital can shield \ (p\). Moving across a period, z_eff. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The shielding effect increases with the number of electrons. Explain Shielding And How It Changes On The Periodic Table.
From www.vrogue.co
Periodic Table Trends Cheat Sheet That I Made I Love vrogue.co Explain Shielding And How It Changes On The Periodic Table The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. Moving across a period, z_eff. Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons.. Explain Shielding And How It Changes On The Periodic Table.
From www.vrogue.co
Periodic Table Trends Concept Map vrogue.co Explain Shielding And How It Changes On The Periodic Table The net positive charge from the nucleus that an electron can “feel” attractions from. Moving across a period, z_eff. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in. The shielding effect increases with the number of electrons between the nucleus and the. Explain Shielding And How It Changes On The Periodic Table.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The Explain Shielding And How It Changes On The Periodic Table Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect increases with the number of electrons between. Explain Shielding And How It Changes On The Periodic Table.
From www.vedantu.com
What are Properties of Elements? Periodic Trends in Properties of Explain Shielding And How It Changes On The Periodic Table The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the. Explain Shielding And How It Changes On The Periodic Table.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The Explain Shielding And How It Changes On The Periodic Table The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the. Explain Shielding And How It Changes On The Periodic Table.
From www.nagwa.com
Question Video Determining How the Atomic Radius and Reactivity of the Explain Shielding And How It Changes On The Periodic Table The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The net positive charge from the nucleus that an electron. Explain Shielding And How It Changes On The Periodic Table.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table Explain Shielding And How It Changes On The Periodic Table The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in. Electrons in an \ (s\) orbital can shield \ (p\). The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the.. Explain Shielding And How It Changes On The Periodic Table.
From socratic.org
How are shielding effect and atomic radius related? Socratic Explain Shielding And How It Changes On The Periodic Table The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in. Moving. Explain Shielding And How It Changes On The Periodic Table.
From app.emaze.com
Untitled on emaze Explain Shielding And How It Changes On The Periodic Table The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. Electrons in an \ (s\) orbital can shield \ (p\). The shielding effect, also known as the screening effect,. Explain Shielding And How It Changes On The Periodic Table.
From ecampusontario.pressbooks.pub
8.7 Periodic Trends and Variation of Properties General Chemistry Explain Shielding And How It Changes On The Periodic Table The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. Electrons in an \ (s\) orbital can shield \ (p\). The shielding effect, also known as the screening effect,. Explain Shielding And How It Changes On The Periodic Table.
From byjus.com
Explain effective nuclear charge shielding effect Factor on which Explain Shielding And How It Changes On The Periodic Table The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. Electrons in an \ (s\) orbital can shield \ (p\). Moving across a period, z_eff. The shielding effect increases with the number of electrons between the nucleus and. Explain Shielding And How It Changes On The Periodic Table.
From pt.slideshare.net
Periodic trends Explain Shielding And How It Changes On The Periodic Table Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in. Moving across a period, z_eff. The shielding effect increases with the number of electrons between. Explain Shielding And How It Changes On The Periodic Table.
From slideplayer.com
Patterns within the periodic table ppt download Explain Shielding And How It Changes On The Periodic Table The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The net positive charge from the nucleus that an electron can “feel” attractions from. Moving across a period, z_eff. Electrons in an \ (s\) orbital can shield \ (p\). The shielding effect, also known as the screening. Explain Shielding And How It Changes On The Periodic Table.
From www.slideshare.net
Chapter 6 Periodic Table Explain Shielding And How It Changes On The Periodic Table The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The net positive charge from the nucleus that an electron can “feel” attractions from. Moving across a period, z_eff.. Explain Shielding And How It Changes On The Periodic Table.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free Explain Shielding And How It Changes On The Periodic Table The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. Electrons in an \ (s\) orbital can shield \ (p\). The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting. Explain Shielding And How It Changes On The Periodic Table.
From socratic.org
How does electronegativity change across a period? Socratic Explain Shielding And How It Changes On The Periodic Table The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in. Moving across a period, z_eff. Electrons in an \ (s\). Explain Shielding And How It Changes On The Periodic Table.
From www.compoundchem.com
Periodicity Trends in the Periodic Table Compound Interest Explain Shielding And How It Changes On The Periodic Table The net positive charge from the nucleus that an electron can “feel” attractions from. Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. Moving across a period, z_eff. The shielding effect increases with. Explain Shielding And How It Changes On The Periodic Table.
From www.slideserve.com
PPT Drill 6 11/11 & 11/12/13 PowerPoint Presentation, free download Explain Shielding And How It Changes On The Periodic Table The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in. Explain Shielding And How It Changes On The Periodic Table.
From www.chem.fsu.edu
Electron Configurations Explain Shielding And How It Changes On The Periodic Table Electrons in an \ (s\) orbital can shield \ (p\). The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the.. Explain Shielding And How It Changes On The Periodic Table.
From www.chemistrylearner.com
Periodic Trends Definition and Properties Explain Shielding And How It Changes On The Periodic Table Electrons in an \ (s\) orbital can shield \ (p\). The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in.. Explain Shielding And How It Changes On The Periodic Table.
From www.ck12.org
Periodic Trends in Atomic Size CK12 Foundation Explain Shielding And How It Changes On The Periodic Table Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. The. Explain Shielding And How It Changes On The Periodic Table.
From www.slideserve.com
PPT The Periodic Properties of the Elements PowerPoint Presentation Explain Shielding And How It Changes On The Periodic Table Electrons in an \ (s\) orbital can shield \ (p\). The net positive charge from the nucleus that an electron can “feel” attractions from. Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting. Explain Shielding And How It Changes On The Periodic Table.
From perso.numericable.fr
The electrons in the inner shells shield the electrons in the outer Explain Shielding And How It Changes On The Periodic Table The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. Moving across a period, z_eff. Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic. Explain Shielding And How It Changes On The Periodic Table.
From learnchemisrty.weebly.com
Periodic Trends Explain Shielding And How It Changes On The Periodic Table Down the periodic table, larger atomic radius causes electrons in valence orbitals to be shielded by core electrons. Electrons in an \ (s\) orbital can shield \ (p\). The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. Moving across a period, z_eff. The net positive charge from the nucleus that an. Explain Shielding And How It Changes On The Periodic Table.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Explain Shielding And How It Changes On The Periodic Table The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. Down. Explain Shielding And How It Changes On The Periodic Table.
From byjus.com
how to calculate the shielding effect Explain Shielding And How It Changes On The Periodic Table Moving across a period, z_eff. Electrons in an \ (s\) orbital can shield \ (p\). The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The net positive charge from the nucleus that an electron can “feel” attractions from. The shielding effect plays a pivotal role in. Explain Shielding And How It Changes On The Periodic Table.
From periodictrendsforhonorschemistry.weebly.com
Atomic Radius Trends of the Periodic Table Explain Shielding And How It Changes On The Periodic Table The shielding effect increases with the number of electrons between the nucleus and the electron in question, resulting in a decrease in the. The shielding effect plays a pivotal role in shaping periodic trends, such as atomic size and ionization energy. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence. Explain Shielding And How It Changes On The Periodic Table.