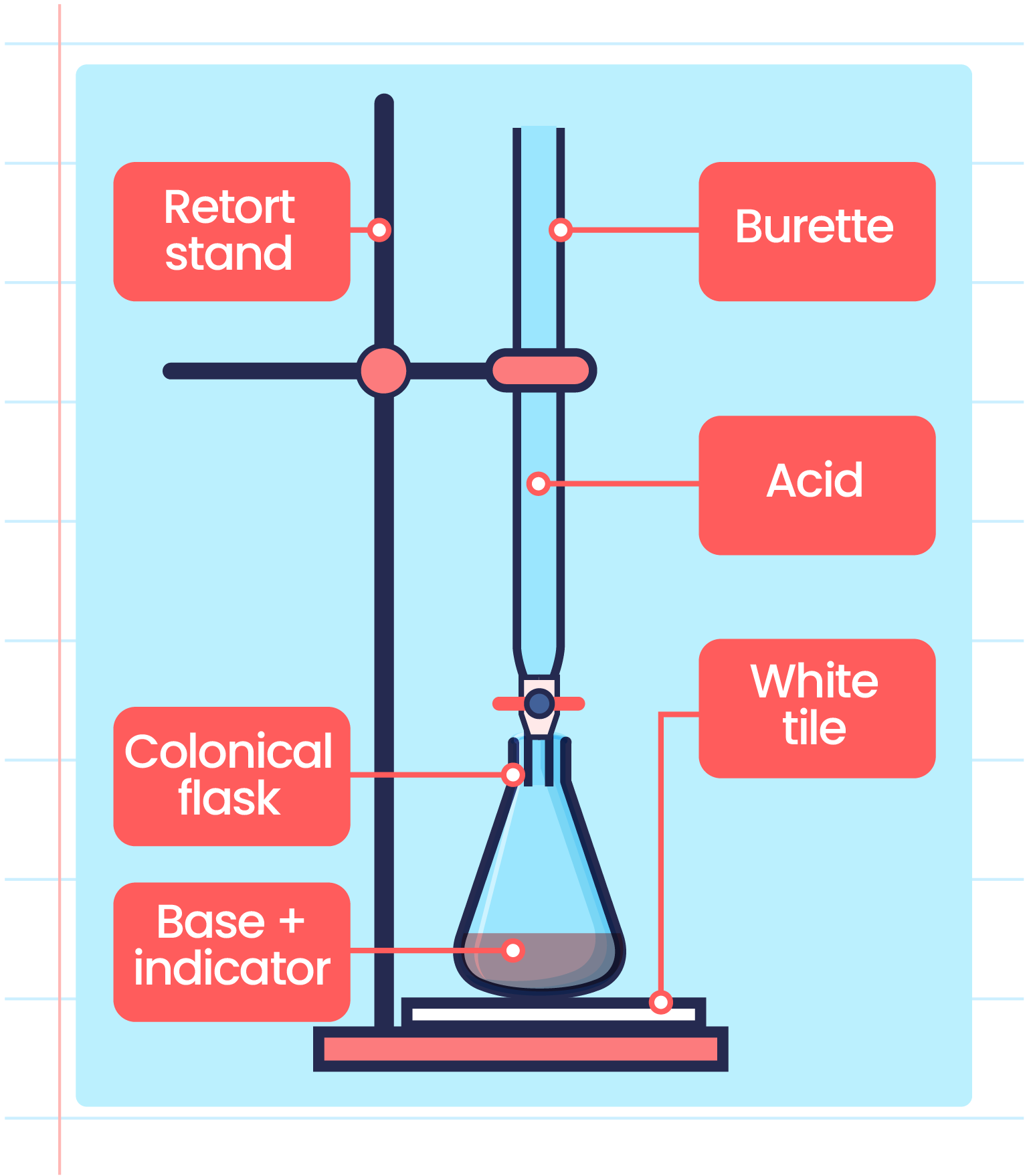
Labeled Titration Setup . A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. Not sure what titration is or what you can do with it? The steps in a titration. In this tutorial, you will find information on titration, including the chemicals that are commonly used and. The titration process can be observed in the video below. Then you are in the right place! Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. Setup for using an indicator to determine the concentration of an unknown through a titration.
from ar.inspiredpencil.com
Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. Then you are in the right place! In this tutorial, you will find information on titration, including the chemicals that are commonly used and. The titration process can be observed in the video below. The steps in a titration. Not sure what titration is or what you can do with it? Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. Setup for using an indicator to determine the concentration of an unknown through a titration.
Titration Setup Diagram
Labeled Titration Setup Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. The steps in a titration. Then you are in the right place! Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. Setup for using an indicator to determine the concentration of an unknown through a titration. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. In this tutorial, you will find information on titration, including the chemicals that are commonly used and. Not sure what titration is or what you can do with it? The titration process can be observed in the video below.
From hubpages.com
Different Methods of Measuring Drug Potency, Concentration, Efficacy Labeled Titration Setup The steps in a titration. Setup for using an indicator to determine the concentration of an unknown through a titration. Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. Not sure what titration is or what you can do with it? Then you are in the right place!. Labeled Titration Setup.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Labeled Titration Setup A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. In this tutorial, you will find information on titration, including the chemicals that are commonly used and. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. Not sure. Labeled Titration Setup.
From iu.pressbooks.pub
Analysis of Acids and Bases by Titration IU East Experimental Labeled Titration Setup The steps in a titration. Not sure what titration is or what you can do with it? Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed. Labeled Titration Setup.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Labeled Titration Setup Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. In this tutorial, you will find information on titration, including the chemicals that are commonly used and. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed. Labeled Titration Setup.
From www.coursehero.com
[Solved] image details Label the titration diagram with the appropriate Labeled Titration Setup A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. Setup for using an indicator to determine the concentration of an unknown through a titration. Not sure what titration is or what you can do with it? Titrations are an analytical technique most commonly used to. Labeled Titration Setup.
From www.numerade.com
SOLVED Label the titration setup below and indicate (place an arrow Labeled Titration Setup Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. In this tutorial, you will find information on titration, including the chemicals that are commonly. Labeled Titration Setup.
From www.savemyexams.co.uk
AcidAlkali Titrations (2.6.3) Edexcel IGCSE Chemistry Revision Notes Labeled Titration Setup Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. The steps in a titration. In this tutorial, you will find information on titration, including. Labeled Titration Setup.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Labeled Titration Setup Then you are in the right place! The steps in a titration. Not sure what titration is or what you can do with it? A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. A white tile is placed under the conical flask while the titration. Labeled Titration Setup.
From www.freepik.com
Premium AI Image A titration setup with a burette and a conical flask Labeled Titration Setup The steps in a titration. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. Then you are in the right place! Setup for using an indicator to determine the concentration of an unknown through a titration. The titration process can be observed in the video. Labeled Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Labeled Titration Setup A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. Titration titration is a quantitative technique that uses a solution of known concentration to. Labeled Titration Setup.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Labeled Titration Setup Then you are in the right place! In this tutorial, you will find information on titration, including the chemicals that are commonly used and. The steps in a titration. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. Setup for using an indicator to determine the concentration of an unknown through a. Labeled Titration Setup.
From ar.inspiredpencil.com
Titration Setup Labeled Titration Setup In this tutorial, you will find information on titration, including the chemicals that are commonly used and. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. Setup for using an indicator to determine the concentration of an unknown through a titration. A white tile is placed under the conical flask while the. Labeled Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Labeled Titration Setup The steps in a titration. Setup for using an indicator to determine the concentration of an unknown through a titration. The titration process can be observed in the video below. Then you are in the right place! Not sure what titration is or what you can do with it? Titration titration is a quantitative technique that uses a solution of. Labeled Titration Setup.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Labeled Titration Setup Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. The titration process can be observed in the video below. Not sure what titration is or what you can do with it? Then you are in the right place! A measured volume of the solution to be titrated, in. Labeled Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Labeled Titration Setup A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. Then you are in the right place! The titration process can be observed in the video below. Not sure what titration is or what you can do with it? A measured volume of the solution to be titrated,. Labeled Titration Setup.
From theedge.com.hk
Chemistry How To Titration The Edge Labeled Titration Setup Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. A white tile is placed under the conical flask while the titration is performed, to make it easier to. Labeled Titration Setup.
From brainly.com
Draw the setup for the titration in Part 1. Label the various parts Labeled Titration Setup Not sure what titration is or what you can do with it? The steps in a titration. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. Then you are in the right place! A measured volume of the solution to be titrated, in this case, colorless aqueous. Labeled Titration Setup.
From www.vrogue.co
Titration Illustration vrogue.co Labeled Titration Setup The steps in a titration. Then you are in the right place! Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. In this tutorial, you will find information on titration, including the chemicals that are commonly used and. A white tile is placed under the conical flask while the titration is performed,. Labeled Titration Setup.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Labeled Titration Setup The titration process can be observed in the video below. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. In this tutorial, you will find information on titration, including the chemicals. Labeled Titration Setup.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Labeled Titration Setup Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. In this tutorial, you will find information on titration, including the chemicals that are commonly used and. The steps in a titration. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the. Labeled Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Labeled Titration Setup In this tutorial, you will find information on titration, including the chemicals that are commonly used and. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. Setup for using an indicator to determine the concentration of an unknown through a titration. A white tile is placed under the conical flask while the. Labeled Titration Setup.
From resource.studiaacademy.com
edexcel_igcse_chemistry_topic15_acidsalkalisandtitrations_004 Labeled Titration Setup Setup for using an indicator to determine the concentration of an unknown through a titration. Not sure what titration is or what you can do with it? The steps in a titration. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. Titration titration is a quantitative technique. Labeled Titration Setup.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Labeled Titration Setup In this tutorial, you will find information on titration, including the chemicals that are commonly used and. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. The steps in a titration. Setup for using an indicator to determine the concentration of an unknown through a. Labeled Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Labeled Titration Setup The steps in a titration. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. Setup for using an indicator to determine the concentration of an unknown through a titration. In this. Labeled Titration Setup.
From sbl1023.blogspot.com
LAB 3 TITRATION Labeled Titration Setup The steps in a titration. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. Setup for using an indicator to determine the concentration of an unknown through a titration. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3. Labeled Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Labeled Titration Setup Not sure what titration is or what you can do with it? A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. Then you are. Labeled Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Labeled Titration Setup The steps in a titration. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. Setup for using an indicator to determine the concentration of an unknown through a titration. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in. Labeled Titration Setup.
From wordwall.net
Titration setup Labelled diagram Labeled Titration Setup Then you are in the right place! A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. Setup for using an indicator to determine the concentration of an unknown. Labeled Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Labeled Titration Setup Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. In this tutorial, you will find information on titration, including the chemicals that are commonly used and. The steps in a titration. Setup for using an indicator to determine the concentration of an unknown through a titration. Then you are in the right. Labeled Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Labeled Titration Setup In this tutorial, you will find information on titration, including the chemicals that are commonly used and. The steps in a titration. Setup for using an indicator to determine the concentration of an unknown through a titration. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. Then you are in the right. Labeled Titration Setup.
From www.sliderbase.com
Titration Labeled Titration Setup In this tutorial, you will find information on titration, including the chemicals that are commonly used and. Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. Setup for using an indicator. Labeled Titration Setup.
From ar.inspiredpencil.com
Titration Diagram Labeled Titration Setup In this tutorial, you will find information on titration, including the chemicals that are commonly used and. Not sure what titration is or what you can do with it? Setup for using an indicator to determine the concentration of an unknown through a titration. A white tile is placed under the conical flask while the titration is performed, to make. Labeled Titration Setup.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Labeled Titration Setup Then you are in the right place! A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. The steps in a titration. In this tutorial, you will find information on titration, including the chemicals that are commonly used and. Titration titration is a quantitative technique that. Labeled Titration Setup.
From atlas.nilebasin.org
Acidbase Titration Setup Phenolphthalein Indicator Vector, 42 OFF Labeled Titration Setup Then you are in the right place! Titrations are an analytical technique most commonly used to calculate the concentration of an unknown (the analyte) with a known (the. The steps in a titration. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. The titration process can be observed in the video below.. Labeled Titration Setup.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Labeled Titration Setup A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is placed in a. Titration titration is a quantitative technique that uses a solution of known concentration to react completely with. The titration process can be observed in the video below. In this tutorial, you will find information on titration,. Labeled Titration Setup.