Osmometry In Polymer . Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. There are four main types of osmometry: There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. Osmometry measures the osmotic strength of a solution using an osmometer. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons.
from www.chegg.com
There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. There are four main types of osmometry: Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Osmometry measures the osmotic strength of a solution using an osmometer.
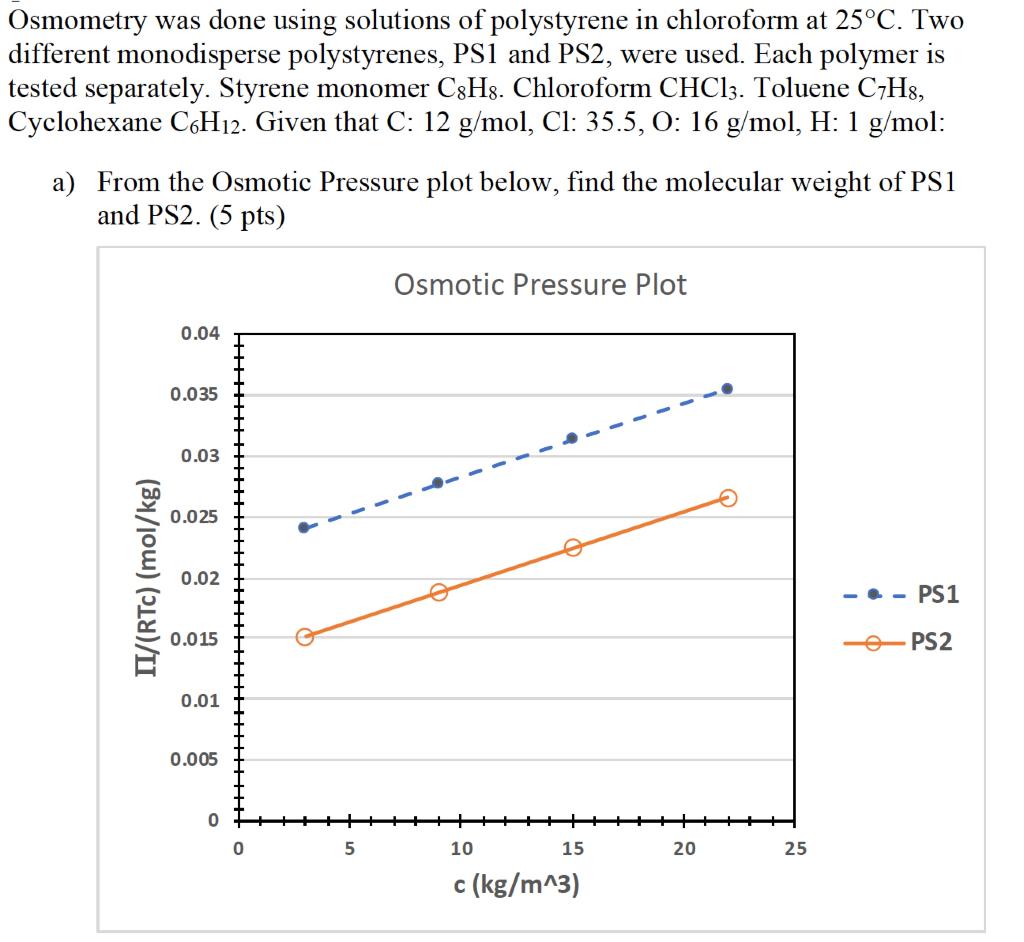
Osmometry was done using solutions of polystyrene in
Osmometry In Polymer There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Osmometry measures the osmotic strength of a solution using an osmometer. Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. There are four main types of osmometry: An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Osmometry In Polymer Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. There are four main types of osmometry: Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations. Osmometry In Polymer.
From www.studypool.com
SOLUTION Polymer physics membrane osmometry Studypool Osmometry In Polymer Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. There are. Osmometry In Polymer.
From www.pathologyinpractice.com
Advanced Instruments introduces the OsmoPRO MAX automated osmometer Osmometry In Polymer Osmometry measures the osmotic strength of a solution using an osmometer. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: There are four main types of osmometry: The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. Membrane osmometry provides valuable insights into polymer. Osmometry In Polymer.
From www.slideserve.com
PPT Recent Developments in Polymer Characterization PowerPoint Osmometry In Polymer An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons. There are four main types of osmometry: Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in. Osmometry In Polymer.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Osmometry In Polymer Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons. There are two principal methods of osmometry that are suitable for determining average. Osmometry In Polymer.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Osmometry In Polymer The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: There. Osmometry In Polymer.
From www.researchgate.net
Agarose gel electrophoresis patterns showing PCR amplification products Osmometry In Polymer The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. Osmometry measures the osmotic strength of a solution using an osmometer. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Osmometry is still of some practical usefulness in polymer science as it is able to. Osmometry In Polymer.
From www.slideserve.com
PPT Recent Developments in Polymer Characterization PowerPoint Osmometry In Polymer Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. There are four. Osmometry In Polymer.
From pubs.rsc.org
Trends in polymeric shape memory hydrogels and hydrogel actuators Osmometry In Polymer An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. Osmometry measures the osmotic strength of a solution using an osmometer. Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer. Osmometry In Polymer.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Osmometry In Polymer There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Osmometry measures the osmotic strength of a solution using an osmometer. There are four main types of osmometry: Membrane osmometry provides valuable insights into polymer characteristics that. Osmometry In Polymer.
From www.chegg.com
Osmometry was done using solutions of polystyrene in Osmometry In Polymer There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. An. Osmometry In Polymer.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Osmometry In Polymer Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons. The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. There are four main types of osmometry: An osmometer is a device for measuring the osmotic strength of a. Osmometry In Polymer.
From www.slideshare.net
Ept 121 lecture membrane osmometry Osmometry In Polymer Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons. Osmometry measures the osmotic strength of a solution using an osmometer. Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. Membrane osmometry ( m n , a 2, χ) osmotic. Osmometry In Polymer.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Osmometry In Polymer Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons. There are four main types of osmometry: The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. Membrane osmometry provides valuable insights into polymer characteristics that are essential for. Osmometry In Polymer.
From www.docsity.com
Osmometry and Light ScatteringPolymer Science and EngineeringLecture Osmometry In Polymer There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. There are four main types of osmometry: Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends. Osmometry In Polymer.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Osmometry In Polymer The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. Osmometry measures the osmotic strength of a solution using an osmometer. There are four main types of osmometry: Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. Membrane osmometry ( m n , a 2,. Osmometry In Polymer.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry In Polymer Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are four main types of osmometry: Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. There are two principal methods of osmometry that are suitable for determining average. Osmometry In Polymer.
From www.mdpi.com
Polymers Free FullText Predicting Characteristics of Dissimilar Osmometry In Polymer Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are four main. Osmometry In Polymer.
From www.flickriver.com
Membrane Osmometry a photo on Flickriver Osmometry In Polymer Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. There are four main types of osmometry: Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a. Osmometry In Polymer.
From www.numerade.com
SOLVED 5 Osmometry (see figure below) is an important method of Osmometry In Polymer Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are four main types of osmometry: Membrane osmometry provides valuable insights into. Osmometry In Polymer.
From www.numerade.com
SOLVED The following data were obtained by membrane osmometry of Osmometry In Polymer An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. There are four main types of osmometry: Membrane osmometry ( m. Osmometry In Polymer.
From slidetodoc.com
Membrane Osmometry Alfredo Clemente CH 392 N Prof Osmometry In Polymer The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. Osmometry measures the osmotic strength of a solution using an osmometer. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are four main types of osmometry:. Osmometry In Polymer.
From www.knauer.net
UHPLC, HPLC, Prep LC, FPLC, SMBC LNP Osmometry KNAUER Osmometry In Polymer The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Membrane osmometry ( m n , a 2, χ). Osmometry In Polymer.
From www.researchgate.net
(PDF) Chain Stoppers in Reversible Supramolecular Polymer Solutions Osmometry In Polymer There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: There are four main types of osmometry: Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. Osmometry is still of some practical usefulness in polymer science as it. Osmometry In Polymer.
From www.indiamart.com
VAPOR PRESSURE OSMOMETER at Rs 1000000/piece Vapor Pressure Osmometer Osmometry In Polymer Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are four main types of osmometry: The three most common forms of directly or indirectly measuring π are vapour pressure osmometry (vpo), freezing point. Osmometry is still of some practical usefulness in polymer science as. Osmometry In Polymer.
From www.amazon.com
VAPOR PRESSURE OSMOMETRY AS A MEANS OF DETERMINING POLYMER MOLECULAR Osmometry In Polymer Osmometry measures the osmotic strength of a solution using an osmometer. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. The three most common forms of directly or indirectly. Osmometry In Polymer.
From www.slideserve.com
PPT Chapter 2. Molecular Weight and Polymer Solutions PowerPoint Osmometry In Polymer Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: There are four main types of osmometry: An osmometer is a device for measuring the osmotic strength of a. Osmometry In Polymer.
From www.chemie.uni-wuerzburg.de
Supramolecular and Polymer Analytical Instrumentation Würthner Group Osmometry In Polymer An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Osmometry measures the osmotic strength of a solution using an osmometer. Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. There are four main types of osmometry: Osmometry is still of some practical usefulness in polymer science. Osmometry In Polymer.
From www.researchgate.net
Types of osmometers. Download Scientific Diagram Osmometry In Polymer Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. Osmometry measures the osmotic strength of a solution using an osmometer. There are four main types of osmometry: Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons. There are two. Osmometry In Polymer.
From www.chegg.com
Osmometry was done using solutions of polystyrene in Osmometry In Polymer There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Osmometry is still of some practical usefulness in polymer science as. Osmometry In Polymer.
From www.concordscientificdevices.com
vaporpressureosmometer Osmometry In Polymer There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: There are four main types of osmometry: Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to. Osmometry In Polymer.
From studylib.net
Membrane Osmometry ( , A , χ) M 2 Osmometry In Polymer Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are four main types of osmometry: There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Membrane osmometry provides valuable insights into polymer characteristics that are essential for. Osmometry In Polymer.
From www.studypool.com
SOLUTION Polymer physics membrane osmometry Studypool Osmometry In Polymer There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Osmometry measures the osmotic. Osmometry In Polymer.
From www.purechemistry.org
Osmometry method to determine molecular weight of polymer Purechemistry Osmometry In Polymer An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. There are four main types of osmometry: Osmometry is still of some practical usefulness in polymer science as it is able to measure large molecules up to about 8000 daltons. There are two principal methods of osmometry that are suitable for determining average molecular. Osmometry In Polymer.
From www.chegg.com
Solved Problem 2 125 pts For membrane osmometry of a Osmometry In Polymer Membrane osmometry provides valuable insights into polymer characteristics that are essential for innovations in polymer science. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are two principal. Osmometry In Polymer.