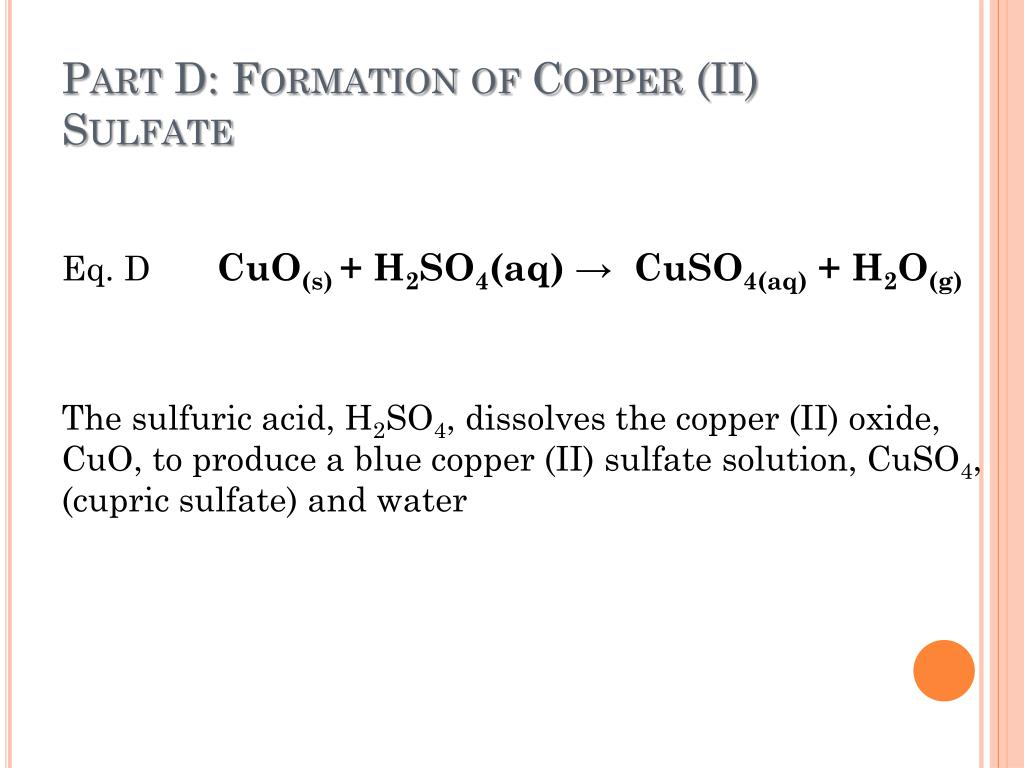
Copper Hydroxide React With Acid . Some basic salts may also form. In fact you get a brown. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Those below hydrogen in the reactivity series don't. Metals above hydrogen in the reactivity series react with acids; Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Finally, zinc metal reduces the hydrated copper. Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer.
from www.slideserve.com
Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. Those below hydrogen in the reactivity series don't. Some basic salts may also form. Finally, zinc metal reduces the hydrated copper. In fact you get a brown. Metals above hydrogen in the reactivity series react with acids; For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2.
PPT Chemical Reactions Copper Reactions PowerPoint Presentation
Copper Hydroxide React With Acid Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Those below hydrogen in the reactivity series don't. Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Some basic salts may also form. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. In fact you get a brown. Metals above hydrogen in the reactivity series react with acids; Finally, zinc metal reduces the hydrated copper. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced.
From www.savemyexams.co.uk
Ligand Substitution Reactions (5.6.4) OCR A Level Chemistry Revision Copper Hydroxide React With Acid Metals above hydrogen in the reactivity series react with acids; Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Finally, zinc metal reduces the hydrated copper. Those below hydrogen in the reactivity series don't. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i). Copper Hydroxide React With Acid.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper Hydroxide React With Acid Metals above hydrogen in the reactivity series react with acids; In fact you get a brown. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Those below hydrogen in the reactivity series don't. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate. Copper Hydroxide React With Acid.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper Hydroxide React With Acid Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. Metals above hydrogen in the reactivity series react with acids; Finally, zinc metal reduces the hydrated copper. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Some basic salts may. Copper Hydroxide React With Acid.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(OH)2 + H2SO4 = CuSO4 + H2O Copper Hydroxide React With Acid Those below hydrogen in the reactivity series don't. Some basic salts may also form. Metals above hydrogen in the reactivity series react with acids; For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate. Copper Hydroxide React With Acid.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper Hydroxide React With Acid Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Metals above hydrogen in the reactivity series react with acids; For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which. Copper Hydroxide React With Acid.
From www.youtube.com
Cu + H2SO4 (Copper + Sulfuric acid) YouTube Copper Hydroxide React With Acid Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. In fact you get a brown. Some basic salts may also form. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which. Copper Hydroxide React With Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Copper Hydroxide React With Acid Some basic salts may also form. Those below hydrogen in the reactivity series don't. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Metals above hydrogen in the reactivity series react with acids; Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't. Copper Hydroxide React With Acid.
From www.youtube.com
Amino Acids 3. Reactions with sodium hydroxide, sodium carbonate Copper Hydroxide React With Acid For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Finally, zinc metal reduces the hydrated copper. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Oxide ions from the copper (ii) oxide and hydrogen ions from the. Copper Hydroxide React With Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide React With Acid Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Some basic salts may also form. For. Copper Hydroxide React With Acid.
From www.youtube.com
Reaction of Copper Oxide With Hydrochloric Acid YouTube Copper Hydroxide React With Acid In fact you get a brown. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. For. Copper Hydroxide React With Acid.
From www.coursehero.com
[Solved] organic chemistry. 3. In the following sequence of reaction A Copper Hydroxide React With Acid Finally, zinc metal reduces the hydrated copper. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. Some basic salts may also form. Those below hydrogen in the reactivity series don't.. Copper Hydroxide React With Acid.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Copper Hydroxide React With Acid In fact you get a brown. Finally, zinc metal reduces the hydrated copper. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Some basic salts may also form. Those below hydrogen in the reactivity series don't. Oxide ions from the copper (ii) oxide and. Copper Hydroxide React With Acid.
From www.thesciencehive.co.uk
Identifying Ions (AQA) — the science sauce Copper Hydroxide React With Acid Metals above hydrogen in the reactivity series react with acids; Those below hydrogen in the reactivity series don't. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Finally, zinc metal reduces the hydrated copper. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i). Copper Hydroxide React With Acid.
From www.dreamstime.com
Double Displacement Reaction Sodium Hydroxide and Copper Sulfate Copper Hydroxide React With Acid Finally, zinc metal reduces the hydrated copper. Metals above hydrogen in the reactivity series react with acids; Those below hydrogen in the reactivity series don't. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Some basic salts may also form. Oxide ions from the. Copper Hydroxide React With Acid.
From fphoto.photoshelter.com
science chemistry precipitation reaction cupric hydroxide Fundamental Copper Hydroxide React With Acid Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Finally, zinc metal reduces the hydrated copper. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer.. Copper Hydroxide React With Acid.
From fphoto.photoshelter.com
science chemistry reaction ligand Fundamental Photographs The Art Copper Hydroxide React With Acid In fact you get a brown. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. Metals. Copper Hydroxide React With Acid.
From polizneuro.weebly.com
Reactivity series of metals polizneuro Copper Hydroxide React With Acid Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. Finally, zinc metal reduces the hydrated copper. Those below hydrogen in the reactivity series don't. Some basic salts may also form. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water.. Copper Hydroxide React With Acid.
From www.goodscience.com.au
AcidBase Reactions Good Science Copper Hydroxide React With Acid For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. In fact you get a brown. Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. Copper oxide dissolves in acid, regenerating. Copper Hydroxide React With Acid.
From www.sciencephoto.com
Copper Hydroxide Precipitate Stock Image C027/9441 Science Photo Copper Hydroxide React With Acid Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Some basic salts may also form. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Finally, zinc metal reduces the hydrated copper. Copper oxide dissolves in acid, regenerating the copper. Copper Hydroxide React With Acid.
From www.nagwa.com
Question Video Using the Series of Chemical Activity to Explain Why Copper Hydroxide React With Acid For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Finally, zinc metal reduces the hydrated copper. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Those below hydrogen in the reactivity series don't. Oxide ions from the. Copper Hydroxide React With Acid.
From www.meritnation.com
What is formed when CuSO4 reacts with Sodium hydroxide solution Also Copper Hydroxide React With Acid Some basic salts may also form. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Those below hydrogen in the reactivity series don't. Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. Copper(ii) ion reacts with stoichiometric quantities of. Copper Hydroxide React With Acid.
From www.slideshare.net
Reaction Of Metal Hydroxides With Acid Copper Hydroxide React With Acid Some basic salts may also form. Those below hydrogen in the reactivity series don't. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Finally, zinc metal reduces the hydrated copper.. Copper Hydroxide React With Acid.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Copper Hydroxide React With Acid Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. In fact you get a brown. Those below hydrogen in the reactivity series don't. Some basic salts may also form. Metals. Copper Hydroxide React With Acid.
From www.youtube.com
Make copper hydroxide with just two compounds YouTube Copper Hydroxide React With Acid Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. Finally, zinc metal reduces the hydrated copper. In fact you get a brown. Some basic salts may also form. Those below hydrogen in the reactivity series don't. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to. Copper Hydroxide React With Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide React With Acid For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Metals above hydrogen in the reactivity series react with acids; Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. In fact you get a brown. Finally, zinc metal reduces the. Copper Hydroxide React With Acid.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Hydroxide React With Acid For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Some basic salts may also form. Finally, zinc metal reduces the hydrated copper. Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any. Copper Hydroxide React With Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide React With Acid Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. Finally, zinc metal reduces the hydrated copper. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Some basic salts may also form. In fact you get a brown. Copper oxide dissolves in. Copper Hydroxide React With Acid.
From chemicaldb.netlify.app
Copper acid or base Copper Hydroxide React With Acid Metals above hydrogen in the reactivity series react with acids; Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. In fact you get a brown. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Some basic salts may also form. Oxide ions from the copper (ii) oxide and. Copper Hydroxide React With Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide React With Acid In fact you get a brown. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Some basic salts may also form. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a. Copper Hydroxide React With Acid.
From www.linstitute.net
CIE A Level Chemistry复习笔记2.2.2 Reactions of Group 2 Oxides, Hydroxides Copper Hydroxide React With Acid Those below hydrogen in the reactivity series don't. Metals above hydrogen in the reactivity series react with acids; In fact you get a brown. Finally, zinc metal reduces the hydrated copper. Some basic salts may also form. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Copper oxide dissolves in acid, regenerating the copper (ii). Copper Hydroxide React With Acid.
From www.youtube.com
Making copper hydroxide YouTube Copper Hydroxide React With Acid For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Finally, zinc metal reduces the hydrated copper. In fact you get a brown. Some basic salts may also form. Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to. Copper Hydroxide React With Acid.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube Copper Hydroxide React With Acid Oxide ions from the copper (ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once. Copper Hydroxide React With Acid.
From brainly.in
complete the following reactions 1)reaction between nitric acid and Copper Hydroxide React With Acid Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Metals above hydrogen in the reactivity series react with acids; Those below hydrogen in the reactivity series don't. Copper oxide dissolves in acid, regenerating the copper (ii) ion, which once again binds to water. Oxide ions from the copper (ii) oxide and hydrogen ions from the. Copper Hydroxide React With Acid.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper Hydroxide React With Acid Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Finally, zinc metal reduces the hydrated copper. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Those below hydrogen in the reactivity series don't. Metals above hydrogen in the reactivity. Copper Hydroxide React With Acid.
From www.slideserve.com
PPT CHAPTER 6 PHYSICAL AND CHEMICAL CHANGES PowerPoint Presentation Copper Hydroxide React With Acid In fact you get a brown. Finally, zinc metal reduces the hydrated copper. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Some basic salts may also form. Those below hydrogen in the reactivity series don't. Metals above hydrogen in the reactivity series react with acids; Copper oxide dissolves in acid, regenerating the copper (ii). Copper Hydroxide React With Acid.