Methyl Silicone Rubber Structure . Modifications to silicone chains include: The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. This characteristic gives silicone its distinctive. The silicone chain exposes organic groups to the outside. Structures and properties of silicones. Furthermore, the methyl groups located on the outside the coil structure can rotate freely. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices.
from www.mdpi.com
The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. The silicone chain exposes organic groups to the outside. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. This characteristic gives silicone its distinctive. Furthermore, the methyl groups located on the outside the coil structure can rotate freely. Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. Structures and properties of silicones. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. Modifications to silicone chains include:
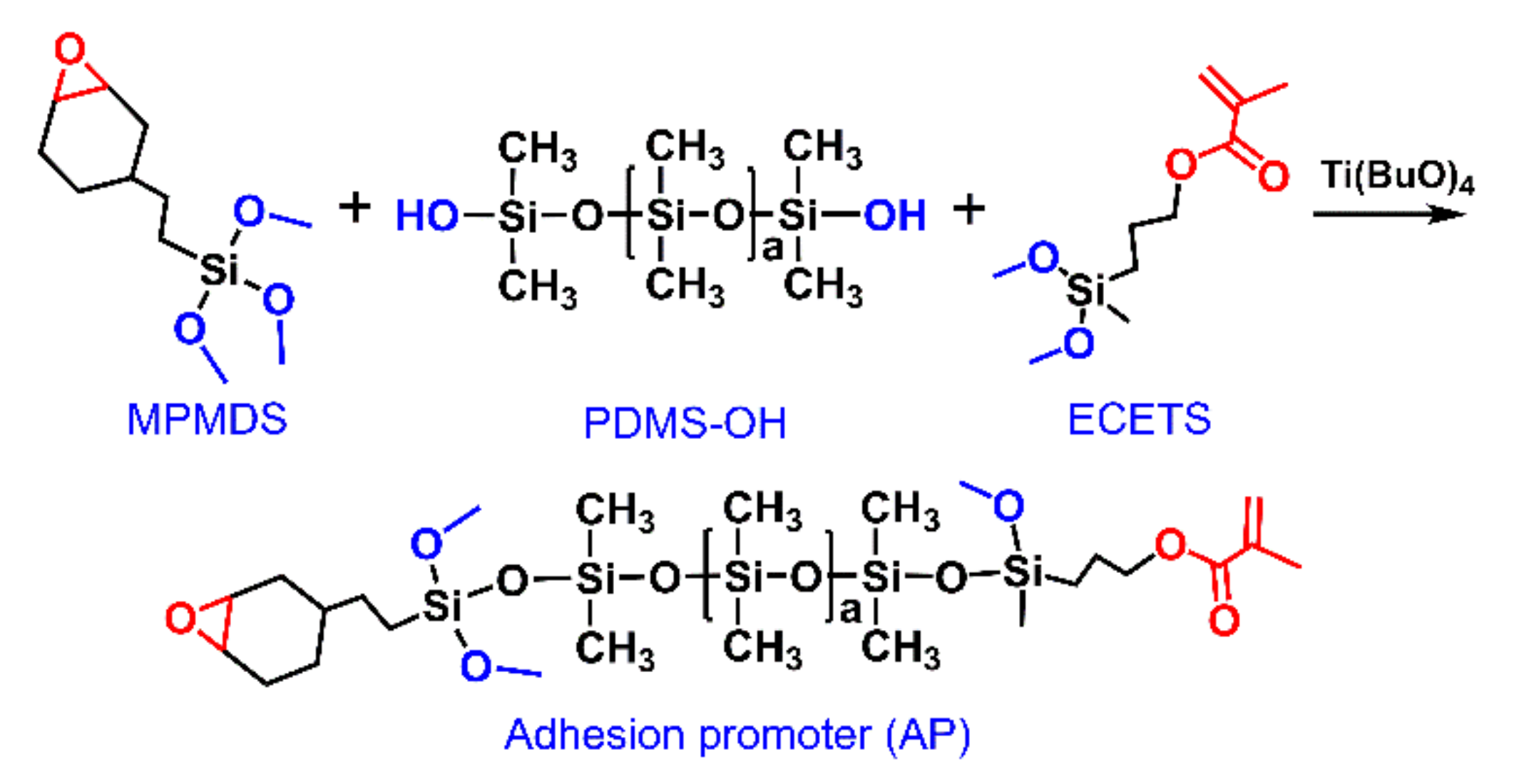
Materials Free FullText Promoters for Improved Adhesion Strength
Methyl Silicone Rubber Structure This characteristic gives silicone its distinctive. The silicone chain exposes organic groups to the outside. The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. This characteristic gives silicone its distinctive. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. Modifications to silicone chains include: Furthermore, the methyl groups located on the outside the coil structure can rotate freely. Structures and properties of silicones. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4.
From www.mdpi.com
Polymers Free FullText The Effect of Various Fillers on the Methyl Silicone Rubber Structure Furthermore, the methyl groups located on the outside the coil structure can rotate freely. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. Structures and properties of silicones. This characteristic gives silicone its distinctive. The organic groups in silicone rubbers may be methyl, vinyl,. Methyl Silicone Rubber Structure.
From www.elastomersilicone.com
methyl vinyl silicone rubber MVQ Silicone 6.5Mpa For Making Tubes And Methyl Silicone Rubber Structure Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. Modifications to silicone chains include: This characteristic gives. Methyl Silicone Rubber Structure.
From pubs.rsc.org
Preparation and characterization of silicone rubber cured via catalyst Methyl Silicone Rubber Structure If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. This. Methyl Silicone Rubber Structure.
From www.mdpi.com
Molecules Free FullText Electric Field Intensity Effects on the Methyl Silicone Rubber Structure Furthermore, the methyl groups located on the outside the coil structure can rotate freely. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. The silicone chain exposes organic groups to the outside. This characteristic gives silicone its distinctive. Structures and. Methyl Silicone Rubber Structure.
From www.semanticscholar.org
[PDF] Polysiloxane Functionalized Carbon Dots and Their CrossLinked Methyl Silicone Rubber Structure Modifications to silicone chains include: The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. The silicone chain exposes organic groups to the outside. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or. Methyl Silicone Rubber Structure.
From pubs.rsc.org
Study on the synthesis and thermal stability of silicone resins Methyl Silicone Rubber Structure If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. Methyl vinyl silicone rubber (mvq) is one of the most commonly used. Methyl Silicone Rubber Structure.
From www.researchgate.net
Scheme of formation of silicone rubber matrix with durably builtin Methyl Silicone Rubber Structure Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. Modifications to silicone. Methyl Silicone Rubber Structure.
From www.mdpi.com
Polymers Free FullText Degradation of Silicone Rubbers as Sealing Methyl Silicone Rubber Structure This characteristic gives silicone its distinctive. Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the. Methyl Silicone Rubber Structure.
From www.alfa-chemical.com
Good Price CAS 67762941 Methyl Vinyl Silicone Rubber for Sale Methyl Silicone Rubber Structure The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. This characteristic gives silicone its distinctive. Furthermore, the methyl groups located on the outside the coil structure can rotate freely. Structures and properties of silicones. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less. Methyl Silicone Rubber Structure.
From www.avkvalves.eu
Insight into the complexity of rubber formulation AVK International Methyl Silicone Rubber Structure This characteristic gives silicone its distinctive. The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. The silicone chain exposes organic groups to the outside. Modifications to silicone chains include: The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. If a small number of the methyl groups in a vmg. Methyl Silicone Rubber Structure.
From www.researchgate.net
The schematic composition and structure of the silicone resin with Methyl Silicone Rubber Structure This characteristic gives silicone its distinctive. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less. Methyl Silicone Rubber Structure.
From www.mdpi.com
Polymers Free FullText Influence of Curing Agent Amount on Methyl Silicone Rubber Structure Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. The astm d1418 standard. Methyl Silicone Rubber Structure.
From www.hubeistarchem.com
Methyl Silicone Resin Methyl Silicone Rubber Structure The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. Structures and properties of. Methyl Silicone Rubber Structure.
From www.shutterstock.com
Silicone Oil (Polydimethylsiloxane, Pdms) Silicone Polymer, Chemical Methyl Silicone Rubber Structure The silicone chain exposes organic groups to the outside. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. Silanes are analogous to hydrocarbons, i.e, the. Methyl Silicone Rubber Structure.
From polymer360.blogspot.com
Silicone rubbers Methyl Silicone Rubber Structure The silicone chain exposes organic groups to the outside. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency. Methyl Silicone Rubber Structure.
From www.mdpi.com
Materials Free FullText Waste Rubber Recycling A Review on the Methyl Silicone Rubber Structure Structures and properties of silicones. Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. Modifications to silicone chains include: Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. The organic groups in silicone rubbers may be. Methyl Silicone Rubber Structure.
From www.researchgate.net
Basic silicone rubber structure. 41 Download Scientific Diagram Methyl Silicone Rubber Structure Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. The silicone chain exposes organic groups to the outside. Modifications to silicone chains include: If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. The astm. Methyl Silicone Rubber Structure.
From www.ecplaza.net
Silicone Rubber/ Methyl RTV107 Zhejiang Hoshine Silicon Industry Co Methyl Silicone Rubber Structure Furthermore, the methyl groups located on the outside the coil structure can rotate freely. Structures and properties of silicones. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. Modifications to silicone chains include: The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. The organic. Methyl Silicone Rubber Structure.
From www.minmetalseast.com
MESIL® MVC Methyl Vinyl Silicone Rubber Compound Methyl Silicone Rubber Structure This characteristic gives silicone its distinctive. Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. Modifications to silicone chains include: Furthermore, the methyl groups located on the outside the coil structure can rotate freely. If a small number of the methyl groups in a vmg are. Methyl Silicone Rubber Structure.
From www.researchgate.net
Scheme 1. The fabrication process of LPBS/methyl vinyl silicone rubber Methyl Silicone Rubber Structure The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. Structures and properties of silicones. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. This characteristic gives silicone its distinctive. Methyl vinyl silicone rubber (mvq) is one of the. Methyl Silicone Rubber Structure.
From www.techemi.com
Silicone Rubber,Methyl RTV 107 CAS NO.70131678 supplier,Silicone Methyl Silicone Rubber Structure This characteristic gives silicone its distinctive. The silicone chain exposes organic groups to the outside. Furthermore, the methyl groups located on the outside the coil structure can rotate freely. Structures and properties of silicones. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. The organic groups in silicone rubbers may. Methyl Silicone Rubber Structure.
From sznewsilicone.com
Room Temperature Vulcanized Methyl Silicone Rubber Methyl Silicone Rubber Structure Modifications to silicone chains include: Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. The silicone chain exposes organic groups to the outside. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to. Methyl Silicone Rubber Structure.
From www.mdpi.com
Polymers Free FullText Synthesis and Characterization of Room Methyl Silicone Rubber Structure Structures and properties of silicones. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. Furthermore, the methyl groups located on the outside the coil structure can rotate freely. The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. This. Methyl Silicone Rubber Structure.
From www.dakenchem.com
Conductive Silicone Rubber Silicone Application From Dakenchem Methyl Silicone Rubber Structure Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. This characteristic gives silicone its distinctive. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with. Methyl Silicone Rubber Structure.
From www.techemi.com
Silicone Rubber,Methyl RTV 107 CAS NO.70131678 supplier,Silicone Methyl Silicone Rubber Structure Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. Furthermore, the methyl groups located on the outside the coil structure can rotate freely. The silicone chain exposes organic groups to the outside. Structures and properties of silicones. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other. Methyl Silicone Rubber Structure.
From www.mdpi.com
Polymers Free FullText The Effect of Various Fillers on the Methyl Silicone Rubber Structure Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. Furthermore, the methyl groups located on the outside the coil structure can rotate freely. Structures and properties of silicones. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic. Methyl Silicone Rubber Structure.
From www.hubeistarchem.com
Methyl Silicone Gum Methyl Silicone Rubber Structure Structures and properties of silicones. Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. Modifications to silicone chains include: This characteristic gives silicone its distinctive. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less. Methyl Silicone Rubber Structure.
From merckindex.rsc.org
Methyl Silicone Resins The Merck Index Online Methyl Silicone Rubber Structure Structures and properties of silicones. The silicone chain exposes organic groups to the outside. Furthermore, the methyl groups located on the outside the coil structure can rotate freely. The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with. Methyl Silicone Rubber Structure.
From ar.inspiredpencil.com
Silicone Structure Methyl Silicone Rubber Structure The silicone chain exposes organic groups to the outside. Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. If a small number of the methyl groups in. Methyl Silicone Rubber Structure.
From www.topsilicone.com
Methyl Phenyl Polysiloxane TPD255 Methyl Silicone Rubber Structure If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. Furthermore, the methyl groups located on the outside the coil structure can rotate freely. Modifications to silicone chains include: The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. The silicone. Methyl Silicone Rubber Structure.
From lookfordiagnosis.com
Silicone Elastomers; Elastomers, Silicone; Rubber Silicone; Silicone Rubber Methyl Silicone Rubber Structure The silicone chain exposes organic groups to the outside. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. This characteristic gives silicone its distinctive. The astm d1418 standard covers a system of general nomenclature for rubber and rubber lattices. Modifications to silicone chains include: Silanes are analogous to hydrocarbons, i.e, the basic building block of. Methyl Silicone Rubber Structure.
From www.researchgate.net
Silicone chemical construction and curing (on the basis of Methyl Silicone Rubber Structure Structures and properties of silicones. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. Furthermore, the methyl groups located on the outside the coil structure can rotate freely. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. Silanes are. Methyl Silicone Rubber Structure.
From classnotes.org.in
Silicones Chemistry, Class 11, pBlock Elements Methyl Silicone Rubber Structure The silicone chain exposes organic groups to the outside. Structures and properties of silicones. The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. Modifications to silicone chains include: Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the ch4 (methane) group, while that for silianes is sih4. Methyl vinyl silicone rubber. Methyl Silicone Rubber Structure.
From www.mdpi.com
Materials Free FullText Promoters for Improved Adhesion Strength Methyl Silicone Rubber Structure The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. This characteristic gives silicone its distinctive. Methyl vinyl silicone rubber (mvq) is one of the most commonly used silicone rubbers, usually initiated with organic peroxides. Modifications to silicone chains include: Furthermore, the methyl groups located on the outside the coil structure can rotate freely. The astm. Methyl Silicone Rubber Structure.
From www.inmr.com
Versatile Chemistry of Silicone Rubber for Electrical Insulation Methyl Silicone Rubber Structure The organic groups in silicone rubbers may be methyl, vinyl, phenyl, or other groups. If a small number of the methyl groups in a vmg are replaced with phenyl groups, the polymer chains have less tendency to pack with. Structures and properties of silicones. This characteristic gives silicone its distinctive. Furthermore, the methyl groups located on the outside the coil. Methyl Silicone Rubber Structure.