Difference Between Hcl And Naoh . What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Both reactants and products are in aqueous. If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. Strong acid (hcl) plus strong base (naoh) giving water. When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: For a buffer to work, both the acid and the base. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat.
from www.numerade.com
Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Strong acid (hcl) plus strong base (naoh) giving water. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? Both reactants and products are in aqueous. For a buffer to work, both the acid and the base.
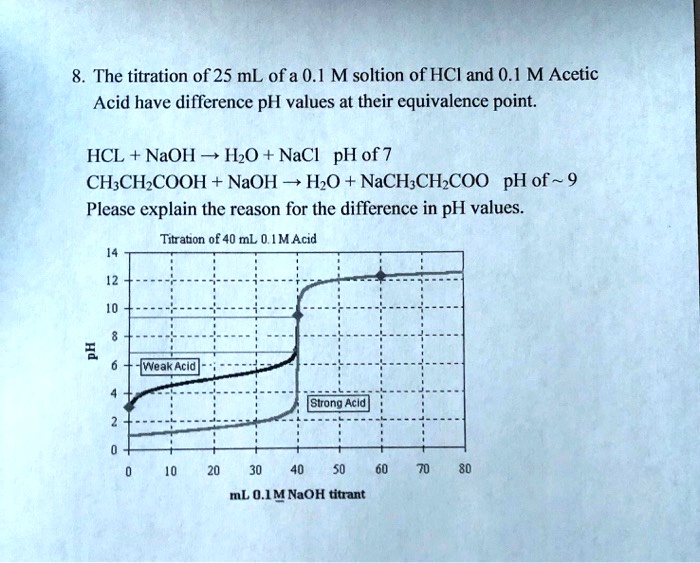
SOLVED The titration of 25 mL of a 0.1 M solution of HCl and 0.1 M
Difference Between Hcl And Naoh If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Strong acid (hcl) plus strong base (naoh) giving water. Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. For a buffer to work, both the acid and the base. Both reactants and products are in aqueous. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners.
From tyrelldesnhlawson.blogspot.com
Ammonium Chloride and Sodium Hydroxide Net Ionic Equation Difference Between Hcl And Naoh What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? For a buffer to work, both the acid and the base. If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. A strong acid and a strong base, such as hcl(aq) and. Difference Between Hcl And Naoh.
From www.youtube.com
Neutralization Reaction between HCl and NaOH Reaction Between HCl and Difference Between Hcl And Naoh If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. Strong acid (hcl) plus strong base (naoh) giving water. Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Both reactants and. Difference Between Hcl And Naoh.
From www.numerade.com
SOLVED Explain the difference in molar heats of neutralization between Difference Between Hcl And Naoh A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. Hcl and naoh are strong acid and strong base respectively and their. Difference Between Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Difference Between Hcl And Naoh What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? Strong acid (hcl) plus strong base (naoh) giving water. When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: Both reactants and products are in aqueous. For a buffer to work, both the acid and the base. A strong acid. Difference Between Hcl And Naoh.
From www.numerade.com
SOLVED (3 points) Consider the graph below Volume of NaOH (mL) If the Difference Between Hcl And Naoh A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. For a buffer to work, both the acid and the base. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. If you mix hcl. Difference Between Hcl And Naoh.
From stock.adobe.com
Acid (HCl), base (NaOH) and salt (NaCl) solutions. Electrolytic Difference Between Hcl And Naoh Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. Both reactants and products are in aqueous. A strong acid and a strong. Difference Between Hcl And Naoh.
From ar.inspiredpencil.com
Naoh Difference Between Hcl And Naoh Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. For a buffer to work, both the acid and the base. If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. A. Difference Between Hcl And Naoh.
From pediaa.com
What is the Difference Between Betaine HCL and Betaine Anhydrous Difference Between Hcl And Naoh For a buffer to work, both the acid and the base. Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Both reactants and products. Difference Between Hcl And Naoh.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Difference Between Hcl And Naoh Strong acid (hcl) plus strong base (naoh) giving water. For a buffer to work, both the acid and the base. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in. Difference Between Hcl And Naoh.
From www.quora.com
What is the difference between HCl and HNO3 in terms of oxidizing power Difference Between Hcl And Naoh Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? Strong acid (hcl) plus strong base (naoh) giving water. Hcl and naoh are strong acid and strong base respectively and their titration curves are. Difference Between Hcl And Naoh.
From byjus.com
24.Why their is difference when naoh and na2co3 are titrated with Difference Between Hcl And Naoh For a buffer to work, both the acid and the base. Both reactants and products are in aqueous. If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +:. Difference Between Hcl And Naoh.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Difference Between Hcl And Naoh What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? For a buffer to work, both the acid and the base. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide. Difference Between Hcl And Naoh.
From eduvark.com
Reaction of HCL and NAOH' 2022 2023 EduVark Difference Between Hcl And Naoh When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in salt and a release. Difference Between Hcl And Naoh.
From studentcomfort26.gitlab.io
Looking Good Chemical Reaction Between Khp And Naoh Hsc Standard Math Difference Between Hcl And Naoh Strong acid (hcl) plus strong base (naoh) giving water. Both reactants and products are in aqueous. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate. Difference Between Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Difference Between Hcl And Naoh Strong acid (hcl) plus strong base (naoh) giving water. Both reactants and products are in aqueous. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. Difference Between Hcl And Naoh.
From www.numerade.com
SOLVED The data for the reaction between HCl and NaOH were as follows Difference Between Hcl And Naoh Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: Both reactants and products are in aqueous. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react. Difference Between Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Difference Between Hcl And Naoh What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. Strong acid (hcl) plus strong base (naoh) giving water. When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and. Difference Between Hcl And Naoh.
From www.numerade.com
SOLVED The titration of 25 mL of a 0.1 M solution of HCl and 0.1 M Difference Between Hcl And Naoh Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? For a buffer to work, both the acid and the base. If you mix hcl and naoh, for example, you will simply neutralize the acid. Difference Between Hcl And Naoh.
From abigailkruwconner.blogspot.com
Enthalpy of Neutralization of Hcl and Naoh Value AbigailkruwConner Difference Between Hcl And Naoh If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of. Difference Between Hcl And Naoh.
From www.toppr.com
Enthalpy of neutralization of HCl by NaOH is 55.84 kJ/mol and by NH Difference Between Hcl And Naoh A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. For a buffer to work, both the acid and the base. Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. What is the. Difference Between Hcl And Naoh.
From www.numerade.com
SOLVED ENTHALPY OF REACTION (HESS'S LAW) DATA Macuun behveer Difference Between Hcl And Naoh For a buffer to work, both the acid and the base. When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: Both reactants and products are in aqueous. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. What is. Difference Between Hcl And Naoh.
From www.numerade.com
SOLVED (3 points) Consider the graph below. Volume of NaOH (mL) If the Difference Between Hcl And Naoh Both reactants and products are in aqueous. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. Hcl and naoh are strong. Difference Between Hcl And Naoh.
From www.numerade.com
SOLVED Title Calculation of Enthalpy of Reaction for Neutralization Difference Between Hcl And Naoh Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. When hydrochloric. Difference Between Hcl And Naoh.
From www.youtube.com
How to Balance NaOH + HCl = NaCl + H2O (Sodium Hydroxide Plus Difference Between Hcl And Naoh If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)?. Difference Between Hcl And Naoh.
From www.numerade.com
SOLVEDReaction NaOH + HClew 47 NaClaw HOHo Question I hypothesis The Difference Between Hcl And Naoh For a buffer to work, both the acid and the base. Strong acid (hcl) plus strong base (naoh) giving water. Both reactants and products are in aqueous. What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in. Difference Between Hcl And Naoh.
From www.numerade.com
SOLVED The heat of neutralisation is highest for the reaction between Difference Between Hcl And Naoh A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. For a buffer to work, both the acid and the base. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. What is the chemical. Difference Between Hcl And Naoh.
From www.coursehero.com
[Solved] Please draw the free energy diagram between HCl and a Difference Between Hcl And Naoh Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Both reactants and products are in aqueous. Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. When hydrochloric acid is dissolved in water, it. Difference Between Hcl And Naoh.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Difference Between Hcl And Naoh Strong acid (hcl) plus strong base (naoh) giving water. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners. When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: Both reactants and products are in aqueous. If you mix hcl. Difference Between Hcl And Naoh.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Difference Between Hcl And Naoh Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a. Difference Between Hcl And Naoh.
From www.toppr.com
Enthalpy of neutralization for both HNO3 and HCl with NaOH is 57.1kJ Difference Between Hcl And Naoh Hcl + naoh = nacl + h₂o + q hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to. Difference Between Hcl And Naoh.
From meetingtarget11.gitlab.io
Ideal Naoh Hcl Balanced Equation What Is The Chemical For Photosynthesis Difference Between Hcl And Naoh Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Strong acid (hcl) plus strong base (naoh) giving water. If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. Both reactants and products. Difference Between Hcl And Naoh.
From www.numerade.com
SOLVED What is the color of phenolphthalein in acid? 2. What is the Difference Between Hcl And Naoh Both reactants and products are in aqueous. When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: For a buffer to work, both the acid and the base. If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer.. Difference Between Hcl And Naoh.
From www.chegg.com
Solved The net ionic equation for the reaction between Difference Between Hcl And Naoh What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: Strong acid (hcl) plus strong base (naoh) giving water. If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral. Difference Between Hcl And Naoh.
From byjus.com
The graph of pH during the titration of NaOH and HCl Difference Between Hcl And Naoh If you mix hcl and naoh, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? Both reactants and products are in aqueous. Strong acid (hcl) plus strong base (naoh) giving water. Hcl and naoh are strong acid. Difference Between Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Difference Between Hcl And Naoh When hydrochloric acid is dissolved in water, it ionizes to form the chloride anion and h +: What is the chemical reaction between hydrochloric acid (hcl) and sodium hydroxide (naoh)? For a buffer to work, both the acid and the base. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution. Difference Between Hcl And Naoh.