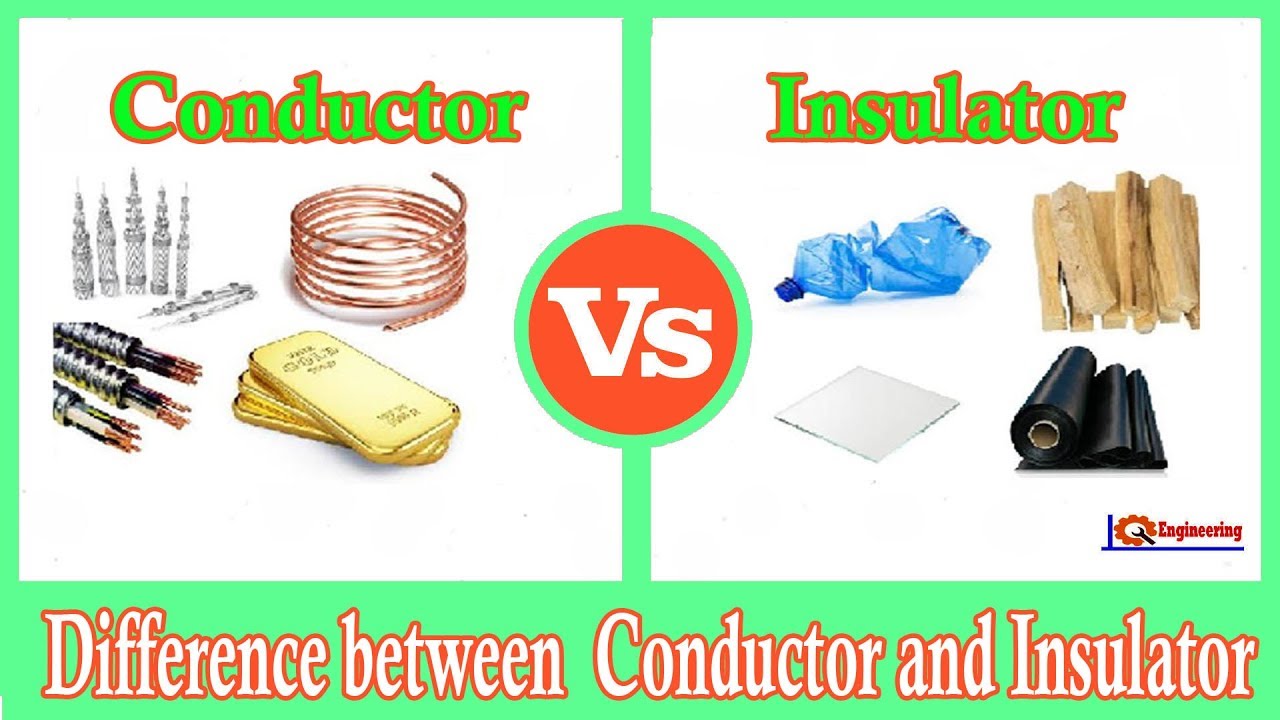
They Are Good Conductors Of Heat And Electricity Ionic Or Covalent . Metals are highly conductive, meaning they are excellent at transferring heat and electricity. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. Covalent or molecular compounds contain atoms held together by covalent bonds. Updated on september 01, 2022. Metals are good conductors because they have free electrons. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. There are four types of crystals: Covalent bonds are highly stable bonds with. Properties and several examples of each type. They are malleable and ductile, meaning metals can. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Silver and copper are the two best conductors of heat and electricity. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and.
from materialfullcrenated.z13.web.core.windows.net
Ionic compounds tend to be crystalline structures with high melting points that are water soluble. They are malleable and ductile, meaning metals can. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. Properties and several examples of each type. Covalent or molecular compounds contain atoms held together by covalent bonds. Updated on september 01, 2022. Silver and copper are the two best conductors of heat and electricity. Metals are highly conductive, meaning they are excellent at transferring heat and electricity. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. Metals are good conductors because they have free electrons.
Conductors Of Heat And Electricity Grade 5
They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Metals are good conductors because they have free electrons. Covalent bonds are highly stable bonds with. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. Silver and copper are the two best conductors of heat and electricity. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Properties and several examples of each type. Metals are good conductors because they have free electrons. They are malleable and ductile, meaning metals can. Covalent or molecular compounds contain atoms held together by covalent bonds. Metals are highly conductive, meaning they are excellent at transferring heat and electricity. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. There are four types of crystals: Updated on september 01, 2022. Ionic compounds tend to be crystalline structures with high melting points that are water soluble.
From www.slideserve.com
PPT HEAT PowerPoint Presentation, free download ID4933138 They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Updated on september 01, 2022. They are malleable and ductile, meaning metals can. Covalent or molecular compounds contain atoms held together by covalent bonds. There are four types of crystals: Metals are good conductors because they have free electrons. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. (1) ionic, (2) metallic, (3) covalent. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.ency123.com
Why are metals good conductors of heat and electricity? They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Properties and several examples of each type. They are malleable and ductile, meaning metals can. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. Covalent bonds are highly stable bonds with. Metals are good conductors because they have free electrons. (1) ionic, (2) metallic, (3) covalent network,. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From animalia-life.club
Thermal Conductivity Of Elements They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Metals are good conductors because they have free electrons. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. They are malleable and ductile, meaning metals can. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. For example, many elements appear shiny, are malleable (able. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.studocu.com
Lesson PLAN IN Conductors OF HEAT AND Electricity LESSON PLAN IN They Are Good Conductors Of Heat And Electricity Ionic Or Covalent For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. There are four types of crystals: They are malleable and ductile, meaning metals can. Silver and copper are the two best conductors of heat and electricity. Ionic compounds tend to be crystalline structures with high melting points that. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From electricitytohitei.blogspot.com
Electricity Good Conductors Of Electricity They Are Good Conductors Of Heat And Electricity Ionic Or Covalent They are malleable and ductile, meaning metals can. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. Updated on september 01, 2022. Covalent. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.slideserve.com
PPT Chapter 7 Properties of Ionic Covalent and Metal Materials They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Metals are good conductors because they have free electrons. Covalent or molecular compounds contain atoms held together by covalent bonds. Silver and copper are the two best conductors of heat and electricity. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. They are malleable and ductile, meaning metals can. Updated on september 01, 2022. Properties and several examples of. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From telgurus.co.uk
Why are metals good conductors of electricity? Science Questionnaire They Are Good Conductors Of Heat And Electricity Ionic Or Covalent For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. Covalent bonds are highly stable bonds with. Metals are good conductors because they have free electrons. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Covalent or molecular compounds contain atoms held together by covalent bonds. Silver. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From blog.stuidapp.com
Everything you need to know about Conductors They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Updated on september 01, 2022. Properties and several examples of each type. Covalent bonds are highly stable bonds with. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. Covalent or molecular compounds contain atoms held together by covalent bonds. Metals are good conductors because they have free. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.slideserve.com
PPT Electrical Electrical Conductors & Insulators PowerPoint They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Properties and several examples of each type. They are malleable and ductile, meaning metals can. Covalent or molecular compounds contain atoms held together by covalent bonds. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. There are four types of crystals: Metals are good conductors because they have free electrons. In fact, many covalent compounds are liquids or gases. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From manhattan8thgradescience.weebly.com
Electricity 8th Grade science They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Covalent or molecular compounds contain atoms held together by covalent bonds. Metals are highly conductive, meaning they are excellent at transferring heat and electricity. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. Ionic compounds tend to be crystalline structures with high melting points that are water. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.slideshare.net
Transmission of heat (ppt) They Are Good Conductors Of Heat And Electricity Ionic Or Covalent For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. Metals are highly conductive, meaning they are excellent at transferring heat and electricity. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. In fact, many covalent compounds are liquids or gases at room temperature, and, in their. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From electricitytohitei.blogspot.com
Electricity Good Conductors Of Electricity They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Properties and several examples of each type. There are four types of crystals: Covalent or molecular compounds contain atoms held together by covalent bonds. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. Silver and copper are the two best conductors of heat and electricity. In fact,. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.youtube.com
10 The good conductor of heat and electricity...... YouTube They Are Good Conductors Of Heat And Electricity Ionic Or Covalent (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Properties and several examples of each type. Metals are good conductors because they have free electrons. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Metals are highly conductive, meaning they are excellent at transferring heat and electricity. They are malleable and ductile, meaning. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From printablemediamoab.z22.web.core.windows.net
Copper Is A Good Conductor They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Updated on september 01, 2022. There are four types of crystals: Ionic compounds tend to be crystalline structures with high melting points that are water soluble. They are malleable and ductile, meaning metals can. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Metals are good conductors because they have free electrons. Metals are highly conductive, meaning they are. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From aracely-has-beard.blogspot.com
Why Are Metals Good Conductors of Both Heat and Electricity Aracely They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Covalent or molecular compounds contain atoms held together by covalent bonds. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. There are four types of crystals: Covalent bonds are highly stable bonds with. Properties and several examples of each type. They are malleable and ductile, meaning metals can. Metals are good conductors because they. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From slideplayer.com
Lesson 3 Conductors and Insulators ppt video online download They Are Good Conductors Of Heat And Electricity Ionic Or Covalent They are malleable and ductile, meaning metals can. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. There are four types of crystals:. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.scienceabc.com
Why Are Metals Good Conductors Of Electricity And Heat? They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Silver and copper are the two best conductors of heat and electricity. Properties and several examples of each type. Metals are good conductors because they have free electrons. Metals are highly conductive, meaning they are excellent at transferring heat and electricity. Covalent or molecular compounds contain atoms held together by covalent bonds. (1) ionic, (2) metallic, (3) covalent network, and. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.aiophotoz.com
What Are Conductors And Insulators Examples Of Conductors And Images They Are Good Conductors Of Heat And Electricity Ionic Or Covalent (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. There are four types of crystals: Covalent or molecular compounds contain atoms held together by covalent bonds. Covalent bonds are highly stable bonds with. Metals are good conductors because they have free electrons. Silver and copper are the two best conductors of heat and electricity. Ionic compounds tend to be. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From design.udlvirtual.edu.pe
Examples Of Good And Bad Conductors Of Heat Design Talk They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Metals are highly conductive, meaning they are excellent at transferring heat and electricity. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. Properties and several examples of each type. Metals are good conductors because they. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From nittygrittyscience.com
Section 1 Electricity Nitty Gritty Science They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Metals are good conductors because they have free electrons. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. (1) ionic, (2) metallic, (3). They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.youtube.com
CONDUCTORS AND INSULATORS SCIENCE EDUCATIONAL VIDEO FOR KIDS YouTube They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Covalent bonds are highly stable bonds with. Covalent or molecular compounds contain atoms held together by covalent bonds. Metals are good conductors because they have free electrons. There are four types of crystals: Updated on september 01, 2022. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and.. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From seanrtmckee.blogspot.com
Good Conductor of Heat SeanrtMckee They Are Good Conductors Of Heat And Electricity Ionic Or Covalent In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. Metals are highly conductive, meaning they are excellent at transferring heat and electricity. Silver and copper are the two best conductors of heat and electricity. They are malleable and ductile, meaning metals can. Updated on september. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From darwintinmelton.blogspot.com
Examples of Conductors of Heat DarwintinMelton They Are Good Conductors Of Heat And Electricity Ionic Or Covalent There are four types of crystals: (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. They are malleable and ductile, meaning metals can. Covalent bonds are highly stable bonds with. Metals are good conductors because they have free electrons. Metals are highly conductive, meaning. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.slideshare.net
Chapter 24 Conduction They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Covalent bonds are highly stable bonds with. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Covalent or molecular compounds. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID1128267 They Are Good Conductors Of Heat And Electricity Ionic Or Covalent For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. Metals are highly conductive, meaning they are excellent at transferring heat and electricity. Covalent bonds are highly stable bonds with. They are malleable and ductile, meaning metals can. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular.. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.youtube.com
Conductors and Insulators Science Experiment Good conductor and bad They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Metals are good conductors because they have free electrons. Covalent or molecular compounds contain atoms held together by covalent bonds. Covalent bonds are highly stable bonds with. Properties and several examples of each type. Silver and copper are the two best conductors of heat and electricity. They are malleable and ductile, meaning metals can. For example, many elements appear shiny,. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.youtube.com
Materials that are Good Conductor of Heat and Electricity YouTube They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Covalent or molecular compounds contain atoms held together by covalent bonds. Updated on september 01, 2022. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and. Covalent bonds are highly stable bonds with. Silver and copper are the. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.youtube.com
DISCUSSING WHY SOME MATERIALS ARE GOOD CONDUCTORS OF HEAT AND They Are Good Conductors Of Heat And Electricity Ionic Or Covalent In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. They are malleable and ductile, meaning metals can. Silver and copper are the two best conductors of heat and electricity. Metals are highly conductive, meaning they are excellent at transferring heat and electricity. (1) ionic, (2). They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.youtube.com
Why metals are good conductors of heat ? YouTube They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Silver and copper are the two best conductors of heat and electricity. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. Covalent bonds are highly stable bonds with. Metals are good conductors because they have free electrons. Updated on september 01, 2022. There are four. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.slideshare.net
Conduction Lesson 2 They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Metals are highly conductive, meaning they are excellent at transferring heat and electricity. They are malleable and ductile, meaning metals can. Metals are good conductors because they have free electrons. Updated on september 01, 2022. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular.. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.slideserve.com
PPT Thermal Energy Transfer PowerPoint Presentation, free download They Are Good Conductors Of Heat And Electricity Ionic Or Covalent In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Updated on september 01, 2022. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires),. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From materialfullcrenated.z13.web.core.windows.net
Conductors Of Heat And Electricity Grade 5 They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Covalent bonds are highly stable bonds with. Covalent or molecular compounds contain atoms held together by covalent bonds. There are four types of crystals: Properties and several examples of each type. Metals are good conductors because they have free electrons. Updated on september 01, 2022. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From www.vrogue.co
The Five Electrical Conductors And Their Uses vrogue.co They Are Good Conductors Of Heat And Electricity Ionic Or Covalent In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. Metals are good conductors because they have free electrons. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Covalent bonds are highly stable bonds with. Silver and copper are the. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From andressegovia5primaria.blogspot.com.es
Clase de 5º Andrés Segovia UNIT 5 HEAT CONDUCTORS AND INSULATORS They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Covalent or molecular compounds contain atoms held together by covalent bonds. Properties and several examples of each type. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic. Covalent bonds are highly stable bonds with. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Metals. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.
From ar.inspiredpencil.com
Conductors Of Electricity They Are Good Conductors Of Heat And Electricity Ionic Or Covalent Metals are good conductors because they have free electrons. There are four types of crystals: Metals are highly conductive, meaning they are excellent at transferring heat and electricity. Properties and several examples of each type. Updated on september 01, 2022. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically. They Are Good Conductors Of Heat And Electricity Ionic Or Covalent.