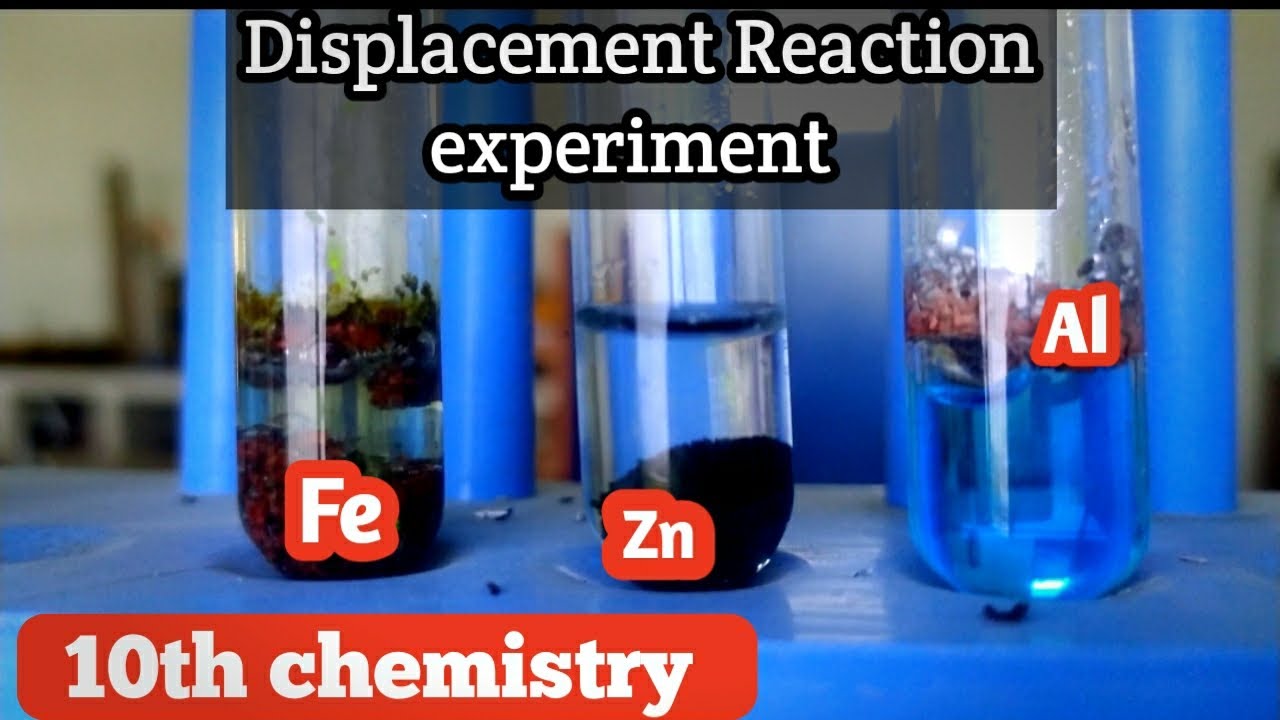
Zinc And Copper Sulfate Displacement Reaction . It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the decomposing zinc metal. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. In a displacement reaction, a less reactive metal is displaced from. However, in the second reaction, the zinc ion is not able to oxidize. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy change for the reaction. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. For example, iron (a metal) reacts with copper sulfate (a salt containing copper). In the first reaction, the copper ion is able to oxidize the zinc metal.
from www.youtube.com
By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy change for the reaction. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. For example, iron (a metal) reacts with copper sulfate (a salt containing copper). When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the decomposing zinc metal. However, in the second reaction, the zinc ion is not able to oxidize. In the first reaction, the copper ion is able to oxidize the zinc metal. In a displacement reaction, a less reactive metal is displaced from. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate.
Experiment on displacement reaction of copper sulphate with iron
Zinc And Copper Sulfate Displacement Reaction However, in the second reaction, the zinc ion is not able to oxidize. However, in the second reaction, the zinc ion is not able to oxidize. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the decomposing zinc metal. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy change for the reaction. For example, iron (a metal) reacts with copper sulfate (a salt containing copper). Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. In the first reaction, the copper ion is able to oxidize the zinc metal. In a displacement reaction, a less reactive metal is displaced from.
From www.alamy.com
Displacement reaction. Image 1 of 2. Displacement reaction between zinc Zinc And Copper Sulfate Displacement Reaction For example, iron (a metal) reacts with copper sulfate (a salt containing copper). If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Zinc And Copper Sulfate Displacement Reaction.
From www.studypool.com
SOLUTION Chemistry lab determining enthalpy change of a reaction Zinc And Copper Sulfate Displacement Reaction In a displacement reaction, a less reactive metal is displaced from. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. However, in the second reaction, the zinc ion is not able to oxidize. If left in the solution for a. Zinc And Copper Sulfate Displacement Reaction.
From www.shalom-education.com
Displacement Reactions GCSE Chemistry Revision Zinc And Copper Sulfate Displacement Reaction When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Zn + cuso4 = cu + znso4 is a single. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
Zinc and Copper Sulphate reaction (Displacement Reaction) Zn and CuSO4 Zinc And Copper Sulfate Displacement Reaction In the first reaction, the copper ion is able to oxidize the zinc metal. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the decomposing zinc metal. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
Experiment on displacement reaction of copper sulphate with iron Zinc And Copper Sulfate Displacement Reaction If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy change for the reaction. When a strip of. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
To Study DISPLACEMENT REACTION of COPPER SULPHATE With Zinc and Iron Zinc And Copper Sulfate Displacement Reaction It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the decomposing zinc metal. In a displacement reaction, a less reactive metal is displaced from. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation. Zinc And Copper Sulfate Displacement Reaction.
From www.pastpapersinside.com
Redox Reaction Past Papers Inside Zinc And Copper Sulfate Displacement Reaction Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the decomposing zinc metal. The experiment reinforces. Zinc And Copper Sulfate Displacement Reaction.
From www.slideshare.net
Replacement reactions Zinc And Copper Sulfate Displacement Reaction For example, iron (a metal) reacts with copper sulfate (a salt containing copper). If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. In the first reaction, the copper ion is able to oxidize the zinc metal. However, in the second reaction, the zinc ion is not able. Zinc And Copper Sulfate Displacement Reaction.
From www.doubtnut.com
Reaction of zinc with solution of copper sulphate, Displacement reacti Zinc And Copper Sulfate Displacement Reaction In a displacement reaction, a less reactive metal is displaced from. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Zinc And Copper Sulfate Displacement Reaction In a displacement reaction, a less reactive metal is displaced from. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. However, in the second reaction, the zinc ion is not able to oxidize. Zn + cuso4 = cu + znso4. Zinc And Copper Sulfate Displacement Reaction.
From www.alamy.com
Displacement reaction. Image 1 of 2. Hand about to add zinc (Zn) metal Zinc And Copper Sulfate Displacement Reaction For example, iron (a metal) reacts with copper sulfate (a salt containing copper). If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time,. Zinc And Copper Sulfate Displacement Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc Fundamental Photographs The Zinc And Copper Sulfate Displacement Reaction However, in the second reaction, the zinc ion is not able to oxidize. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs,. Zinc And Copper Sulfate Displacement Reaction.
From www.scribd.com
Finding The Enthalpy of The Displacement Reaction of Zinc and Copper Zinc And Copper Sulfate Displacement Reaction However, in the second reaction, the zinc ion is not able to oxidize. In the first reaction, the copper ion is able to oxidize the zinc metal. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy change for the. Zinc And Copper Sulfate Displacement Reaction.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0146 Science Zinc And Copper Sulfate Displacement Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy change for the reaction. When a strip of zinc. Zinc And Copper Sulfate Displacement Reaction.
From edu-rsc-org-s.webvpn.bjmu.doc110.com
Exothermic metal displacement reactions Experiment RSC Education Zinc And Copper Sulfate Displacement Reaction If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy change for the reaction. However, in the second. Zinc And Copper Sulfate Displacement Reaction.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc And Copper Sulfate Displacement Reaction By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy change for the reaction. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. However, in the second. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
single displacement reaction of Copper from Copper Sulphate Solution by Zinc And Copper Sulfate Displacement Reaction In a displacement reaction, a less reactive metal is displaced from. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate.. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
Single Displacement Reaction between Copper Sulphate solution and Zinc Zinc And Copper Sulfate Displacement Reaction When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. For example, iron (a metal) reacts with copper sulfate (a salt containing copper). In a displacement reaction, a less reactive metal is displaced from. If left in the solution for a. Zinc And Copper Sulfate Displacement Reaction.
From www.toppr.com
What happens when a piece of zinc metal is added to copper sulphate Zinc And Copper Sulfate Displacement Reaction In the first reaction, the copper ion is able to oxidize the zinc metal. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
DISPLACEMENT OF COPPER SULPHATE WITH ZINC SHOWING THE DISPLACEMENT Zinc And Copper Sulfate Displacement Reaction For example, iron (a metal) reacts with copper sulfate (a salt containing copper). Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. In a displacement reaction, a less reactive metal is displaced from. However, in the second reaction, the zinc ion is. Zinc And Copper Sulfate Displacement Reaction.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Redox (II) Oxidation and Reduction Zinc And Copper Sulfate Displacement Reaction However, in the second reaction, the zinc ion is not able to oxidize. In a displacement reaction, a less reactive metal is displaced from. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. The experiment reinforces ideas about energy changes. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
Reaction of zinc metal with copper sulphate solution class 8th Zinc And Copper Sulfate Displacement Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the decomposing zinc metal. When a strip of zinc metal is placed into. Zinc And Copper Sulfate Displacement Reaction.
From www.teachoo.com
Displacement Reaction and Reactivity Series Concepts Zinc And Copper Sulfate Displacement Reaction When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. For example, iron (a metal) reacts with copper sulfate (a. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
The reaction between Zinc and Copper Sulfate YouTube Zinc And Copper Sulfate Displacement Reaction When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. However, in the second reaction, the zinc ion is not able to oxidize. In a displacement reaction, a less reactive metal is displaced from. The experiment reinforces ideas about energy changes. Zinc And Copper Sulfate Displacement Reaction.
From www.sciencephoto.com
Zinc, Copper Sulphate Reaction, 2 of 2 Stock Image C030/7891 Zinc And Copper Sulfate Displacement Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. In the first reaction, the copper ion is able to oxidize the zinc metal. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
Redox reaction from dissolving zinc in copper sulfate Chemistry Zinc And Copper Sulfate Displacement Reaction If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. However, in the second reaction, the zinc ion is not able to oxidize. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
Zinc (Zn) reaction with Copper Sulphate (CuSO4) Displacement Reaction Zinc And Copper Sulfate Displacement Reaction Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. By adding an excess of zinc powder to a known amount. Zinc And Copper Sulfate Displacement Reaction.
From en.ppt-online.org
Metals online presentation Zinc And Copper Sulfate Displacement Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. In the first reaction, the copper ion is able to oxidize the zinc metal. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. However, in the. Zinc And Copper Sulfate Displacement Reaction.
From www.science-revision.co.uk
Enthalpy changes in solution Zinc And Copper Sulfate Displacement Reaction It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the decomposing zinc metal. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Zinc And Copper Sulfate Displacement Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. In a displacement reaction, a less reactive metal is displaced from. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to. Zinc And Copper Sulfate Displacement Reaction.
From www.youtube.com
Showing displacement reaction between copper sulphate and zinc YouTube Zinc And Copper Sulfate Displacement Reaction In the first reaction, the copper ion is able to oxidize the zinc metal. However, in the second reaction, the zinc ion is not able to oxidize. In a displacement reaction, a less reactive metal is displaced from. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one. Zinc And Copper Sulfate Displacement Reaction.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0148 Science Zinc And Copper Sulfate Displacement Reaction In a displacement reaction, a less reactive metal is displaced from. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a. Zinc And Copper Sulfate Displacement Reaction.
From www.science-revision.co.uk
Displacement reactions Zinc And Copper Sulfate Displacement Reaction It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the decomposing zinc metal. For example, iron (a metal) reacts with copper sulfate (a salt containing copper). When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure. Zinc And Copper Sulfate Displacement Reaction.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc And Copper Sulfate Displacement Reaction By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy change for the reaction. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. When a strip of zinc. Zinc And Copper Sulfate Displacement Reaction.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Zinc And Copper Sulfate Displacement Reaction It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the decomposing zinc metal. However, in the second reaction, the zinc ion is not able to oxidize. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring. Zinc And Copper Sulfate Displacement Reaction.