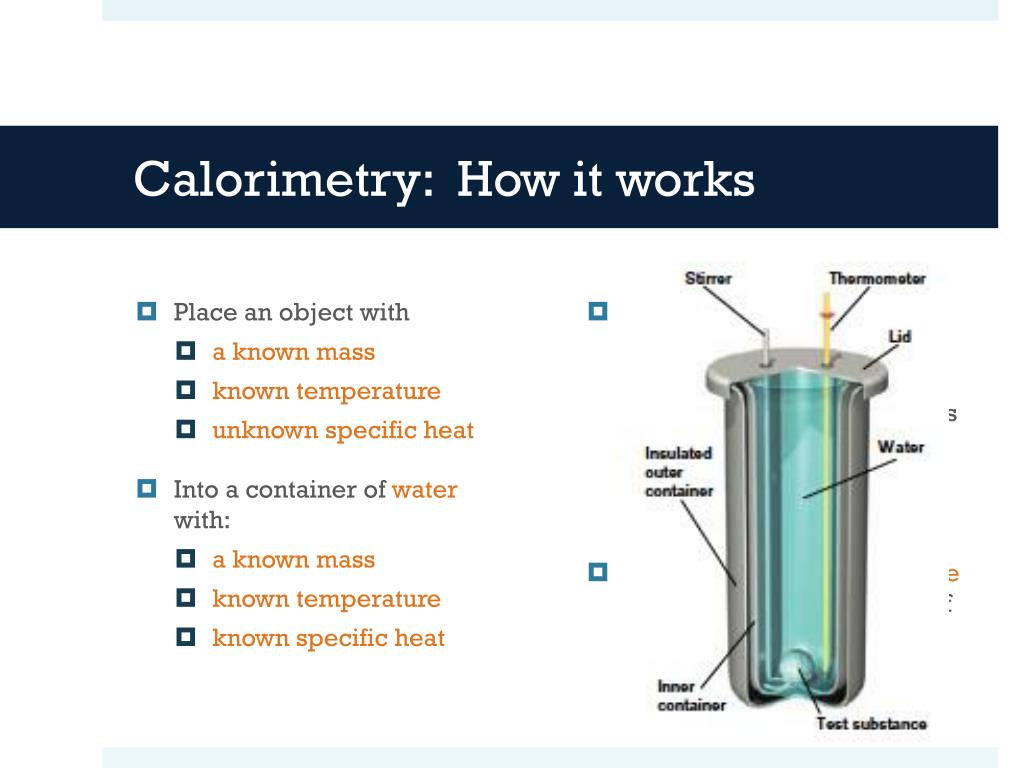
Calorimetry Facts . Calorimetry is the science of measuring the heat of chemical reactions or physical changes. The bomb calorimeter has an enclosure in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the science of measuring heat transfer in physical and chemical processes. For example, when an exothermic. By knowing the change in heat, it can be. Calorimetry is the science of measuring the heat transfer associated with chemical reactions or physical changes. It involves determining the amount of energy. This method is crucial for. It plays a crucial role in determining heat capacity and.
from www.slideserve.com
This method is crucial for. It involves determining the amount of energy. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The bomb calorimeter has an enclosure in. Calorimetry is the science of measuring heat transfer in physical and chemical processes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. For example, when an exothermic. It plays a crucial role in determining heat capacity and. Calorimetry is the science of measuring the heat transfer associated with chemical reactions or physical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
PPT 10.3.1 Specific Heat and Calorimetry PowerPoint Presentation
Calorimetry Facts Calorimetry is the science of measuring the heat of chemical reactions or physical changes. It involves determining the amount of energy. For example, when an exothermic. Calorimetry is the science of measuring heat transfer in physical and chemical processes. Calorimetry is the science of measuring the heat transfer associated with chemical reactions or physical changes. Calorimetry is the science of measuring the heat of chemical reactions or physical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in heat, it can be. This method is crucial for. It plays a crucial role in determining heat capacity and. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The bomb calorimeter has an enclosure in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Facts Calorimetry is the science of measuring the heat transfer associated with chemical reactions or physical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. It involves determining the amount of energy. It plays a crucial role in determining heat capacity and. Calorimetry is the science of. Calorimetry Facts.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 Calorimetry Facts Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. It involves determining the amount of energy. Calorimetry is the science of measuring heat transfer in physical and chemical. Calorimetry Facts.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry Facts This method is crucial for. Calorimetry is the science of measuring the heat transfer associated with chemical reactions or physical changes. It plays a crucial role in determining heat capacity and. It involves determining the amount of energy. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials.. Calorimetry Facts.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Facts It plays a crucial role in determining heat capacity and. It involves determining the amount of energy. For example, when an exothermic. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. This method is crucial for. A calorimeter is a device used to measure the. Calorimetry Facts.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo Calorimetry Facts The bomb calorimeter has an enclosure in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is the science of measuring heat. Calorimetry Facts.
From www.pinterest.com
Class 10 ICSE Physics flash card for Calorimetry Units. . . physics Calorimetry Facts By knowing the change in heat, it can be. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is the science of measuring heat transfer in physical and chemical processes. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical. Calorimetry Facts.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Facts This method is crucial for. Calorimetry is the science of measuring the heat transfer associated with chemical reactions or physical changes. The bomb calorimeter has an enclosure in. By knowing the change in heat, it can be. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical. Calorimetry Facts.
From slideplayer.com
Measuring Food Energy by Calorimetry ppt download Calorimetry Facts Calorimetry is the science of measuring heat transfer in physical and chemical processes. It involves determining the amount of energy. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimetry Facts.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Facts The bomb calorimeter has an enclosure in. Calorimetry is the science of measuring heat transfer in physical and chemical processes. By knowing the change in heat, it can be. Calorimetry is the science of measuring the heat of chemical reactions or physical changes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features. Calorimetry Facts.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry Facts It plays a crucial role in determining heat capacity and. By knowing the change in heat, it can be. It involves determining the amount of energy. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of. Calorimetry Facts.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Facts Calorimetry is the science of measuring the heat transfer associated with chemical reactions or physical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The bomb calorimeter. Calorimetry Facts.
From dxojkdaez.blob.core.windows.net
Calorimetry Physics Examples at Jeremy Wetmore blog Calorimetry Facts For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the science of measuring the heat of chemical reactions or physical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of. Calorimetry Facts.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Facts It involves determining the amount of energy. Calorimetry is the science of measuring heat transfer in physical and chemical processes. Calorimetry is the science of measuring the heat transfer associated with chemical reactions or physical changes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.. Calorimetry Facts.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Facts Calorimetry is the science of measuring the heat of chemical reactions or physical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the science of measuring heat. Calorimetry Facts.
From www.slideserve.com
PPT 10.3.1 Specific Heat and Calorimetry PowerPoint Presentation Calorimetry Facts Calorimetry is the science of measuring heat transfer in physical and chemical processes. It plays a crucial role in determining heat capacity and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in heat, it can be. Calorimetry is the science of measuring the heat transfer. Calorimetry Facts.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Facts Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the science of measuring heat transfer in physical and chemical processes. By knowing the change in heat,. Calorimetry Facts.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Facts This method is crucial for. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. It plays a crucial role in determining heat capacity and. The bomb calorimeter has an enclosure in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical. Calorimetry Facts.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Facts It involves determining the amount of energy. This method is crucial for. It plays a crucial role in determining heat capacity and. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the science of measuring heat transfer in physical and chemical processes. Calorimeter, device for measuring the heat developed during. Calorimetry Facts.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Facts This method is crucial for. Calorimetry is the science of measuring the heat of chemical reactions or physical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in heat, it can be. Calorimetry is a branch of science concerned with measuring a body’s state in. Calorimetry Facts.
From studylib.net
Calorimetry Calorimetry Facts This method is crucial for. It plays a crucial role in determining heat capacity and. Calorimetry is the science of measuring heat transfer in physical and chemical processes. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a. Calorimetry Facts.
From joiolgume.blob.core.windows.net
Calorimetry Heat Calculations at Brenda Salzer blog Calorimetry Facts It involves determining the amount of energy. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The bomb calorimeter has an enclosure in. Calorimetry is the science of measuring the heat transfer associated with chemical reactions or physical changes. Calorimetry is the science of measuring heat transfer in physical and. Calorimetry Facts.
From www.studocu.com
Calorimetry Examples in Chemistry Chapter 9 Studocu Calorimetry Facts A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It plays a crucial role in determining heat capacity and. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. Calorimetry is the science of measuring the heat of. Calorimetry Facts.
From chem.libretexts.org
6.5 Constant Volume Calorimetry Measuring ΔU for Chemical Reactions Calorimetry Facts It involves determining the amount of energy. The bomb calorimeter has an enclosure in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the science of measuring heat transfer in physical and chemical processes. By knowing the change in heat, it can be. This method is crucial for.. Calorimetry Facts.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Facts The bomb calorimeter has an enclosure in. By knowing the change in heat, it can be. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. This method is crucial for. Calorimetry is the science of measuring the heat of chemical reactions or physical changes. For example, when. Calorimetry Facts.
From dxofzsmrb.blob.core.windows.net
Equation Used For Calorimetry at Devin Johnson blog Calorimetry Facts Calorimetry is the science of measuring the heat transfer associated with chemical reactions or physical changes. It involves determining the amount of energy. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. This method is crucial for. Calorimetry is a branch of science concerned with measuring a body’s state in terms of. Calorimetry Facts.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Facts It involves determining the amount of energy. It plays a crucial role in determining heat capacity and. Calorimetry is the science of measuring heat transfer in physical and chemical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released. Calorimetry Facts.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Calorimetry Facts For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is the process of measuring the amount of heat released or. Calorimetry Facts.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimetry Facts A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It plays a crucial role in determining heat capacity and. Calorimetry is the science of measuring the heat of chemical reactions or physical changes. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. Calorimetry Facts.
From dxoazusse.blob.core.windows.net
Food Calorimetry Equation at Jesse Freeman blog Calorimetry Facts Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. It plays a crucial role in determining heat capacity and. This method is crucial for. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring. Calorimetry Facts.
From users.highland.edu
Calorimetry Calorimetry Facts Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. It involves determining the amount of energy. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The bomb calorimeter has an enclosure in. Calorimetry is the science of measuring the heat transfer associated. Calorimetry Facts.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimetry Facts A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. This method is crucial for. Calorimetry is a branch of science concerned with measuring a body’s state in terms. Calorimetry Facts.
From jiahui-fahrenheitnanime.blogspot.com
Calorimetry Worksheet Calorimetry Facts Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The bomb calorimeter has an enclosure in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state. Calorimetry Facts.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry Facts Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. This method is crucial for. Calorimetry is the science of measuring the heat transfer associated with chemical reactions or physical changes. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical,. Calorimetry Facts.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Facts It plays a crucial role in determining heat capacity and. For example, when an exothermic. By knowing the change in heat, it can be. Calorimetry is the science of measuring the heat transfer associated with chemical reactions or physical changes. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is. Calorimetry Facts.
From www.studocu.com
Chem 9 calorimetry Chem 9. Awill be Topic 19 Calorimetry (5 220231 Calorimetry Facts Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. This method is crucial for. Calorimetry is the science of measuring heat transfer in. Calorimetry Facts.