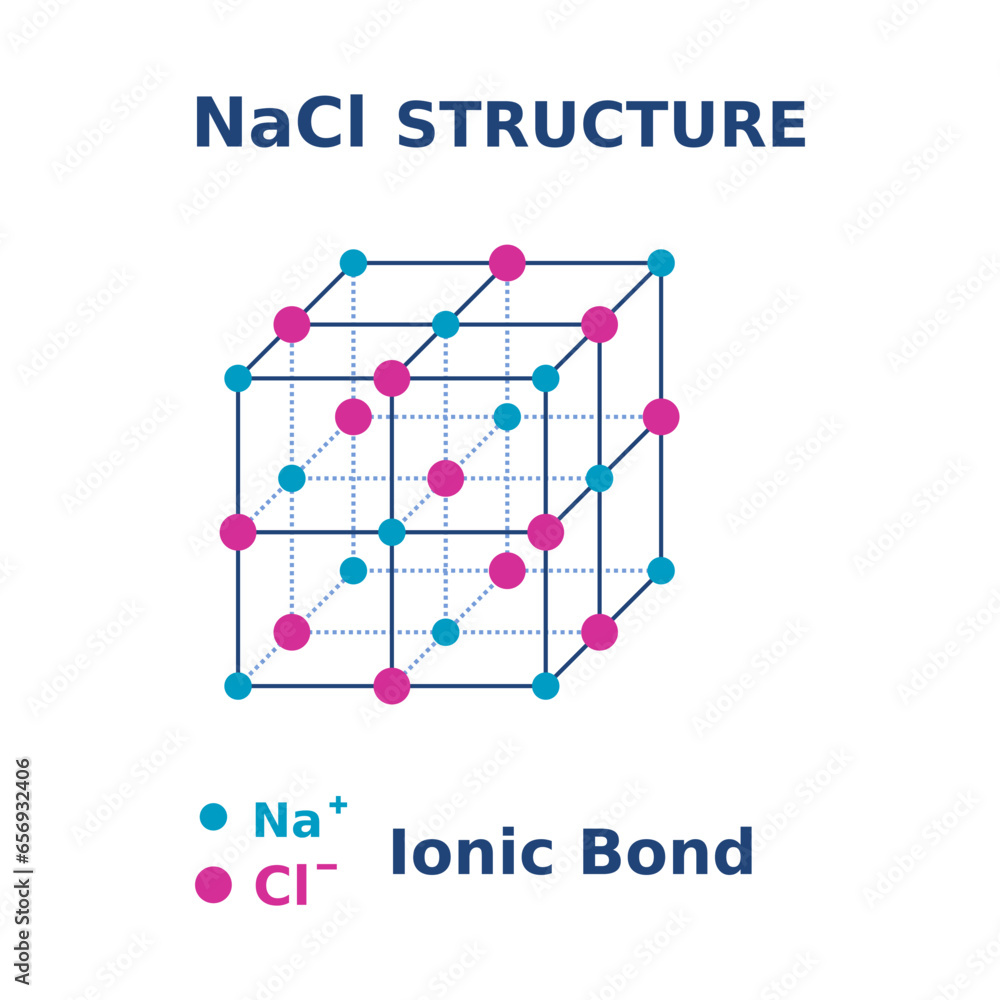
Chloride Ion Unit Cell . We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. Note that there is no lattice point in the center. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula, nacl. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. The unit cell for sodium chloride is shown in figure 1. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. Based on this we can predict that the chloride ions will.
from stock.adobe.com
Note that there is no lattice point in the center. Based on this we can predict that the chloride ions will. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula, nacl. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. The unit cell for sodium chloride is shown in figure 1. We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride.
Vetor de NaCl structure. Sodium chloride molecule. Salt crystal
Chloride Ion Unit Cell Note that there is no lattice point in the center. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. The unit cell for sodium chloride is shown in figure 1. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula, nacl. Based on this we can predict that the chloride ions will. Note that there is no lattice point in the center. One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride.
From www.youtube.com
Ionic Unit Cell YouTube Chloride Ion Unit Cell One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal.. Chloride Ion Unit Cell.
From commons.wikimedia.org
FileSodiumchlorideunitcell3Dionic.png Wikimedia Commons Chloride Ion Unit Cell The unit cell for sodium chloride is shown in figure 1. We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. A unit cell is the smallest unit. Chloride Ion Unit Cell.
From socratic.org
How would you describe the arrangement of sodium ions and chloride ions Chloride Ion Unit Cell A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. Based on this we can predict that the chloride ions will. We will look at the ionic structures in the. Chloride Ion Unit Cell.
From byjus.com
The co ordination number of a chlorine ion in a sodium chloride lattice Chloride Ion Unit Cell One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. The unit cell for sodium chloride is shown in figure 1. Based on this we can predict that the chloride ions will. A unit cell is the smallest unit that when stacked together repeatedly without any gaps. Chloride Ion Unit Cell.
From www.numerade.com
The sodium chloride has a crystal structure (a unit cell) as described Chloride Ion Unit Cell The unit cell for sodium chloride is shown in figure 1. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. We will look at the ionic structures in the next section, and. Chloride Ion Unit Cell.
From chemistry.stackexchange.com
chemistry How do you determine a chemical formula given the Chloride Ion Unit Cell The unit cell for sodium chloride is shown in figure 1. Based on this we can predict that the chloride ions will. We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. The unit cell contains four sodium ions and four chloride ions, giving the. Chloride Ion Unit Cell.
From www.numerade.com
SOLVED Solic strontium chloride has crystal structure With the cubic Chloride Ion Unit Cell The unit cell for sodium chloride is shown in figure 1. Based on this we can predict that the chloride ions will. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula, nacl. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. We. Chloride Ion Unit Cell.
From www.slideserve.com
PPT Crystallography and Structure PowerPoint Presentation ID397496 Chloride Ion Unit Cell We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. A unit cell is the smallest unit that when stacked together repeatedly. Chloride Ion Unit Cell.
From www.numerade.com
SOLVEDList the point coordinates of both the sodium (Na) and chlorine Chloride Ion Unit Cell With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to. Chloride Ion Unit Cell.
From mavink.com
Unit Cell Of Sodium Chloride Chloride Ion Unit Cell Note that there is no lattice point in the center. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. One cesium ion and one chloride ion are present per. Chloride Ion Unit Cell.
From saylordotorg.github.io
Structures of Simple Binary Compounds Chloride Ion Unit Cell The unit cell for sodium chloride is shown in figure 1. Note that there is no lattice point in the center. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula,. Chloride Ion Unit Cell.
From ar.inspiredpencil.com
Atomic Structure Of Sodium Chloride Chloride Ion Unit Cell One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by. Chloride Ion Unit Cell.
From www.chegg.com
Solved Show how the sodium chloride unit cell contains a Chloride Ion Unit Cell We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula, nacl. The unit cell for sodium chloride is shown in figure 1. A unit cell is. Chloride Ion Unit Cell.
From wisc.pb.unizin.org
Ionic Crystals and Unit Cell Stoichiometry (M11Q6) UWMadison Chloride Ion Unit Cell The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula, nacl. Note that there is no lattice point in the center. We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. A unit cell is the. Chloride Ion Unit Cell.
From byjus.com
43. Caesium and chloride ion are in contact along the body diagonal in Chloride Ion Unit Cell A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce. Chloride Ion Unit Cell.
From chem.libretexts.org
12.3 Structures of Simple Binary Compounds Chemistry LibreTexts Chloride Ion Unit Cell Based on this we can predict that the chloride ions will. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. The unit cell for sodium chloride is shown in. Chloride Ion Unit Cell.
From www.slideserve.com
PPT Figure 16.9 Three cubic unit cells and the corresponding Chloride Ion Unit Cell We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry. Chloride Ion Unit Cell.
From askfilo.com
Each unit cell of NaCℓ consists of 14 Chloride ions and Filo Chloride Ion Unit Cell Note that there is no lattice point in the center. Based on this we can predict that the chloride ions will. One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. We will look at the ionic structures in the next section, and here focus on the. Chloride Ion Unit Cell.
From www.youtube.com
Sodium Chloride Unit Cell YouTube Chloride Ion Unit Cell With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula, nacl. We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to. Chloride Ion Unit Cell.
From www.alamy.com
Crystal structure of sodium chloride, illustration Stock Photo Alamy Chloride Ion Unit Cell The unit cell for sodium chloride is shown in figure 1. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. A unit cell is. Chloride Ion Unit Cell.
From chemistryskills.com
Structure of Sodium Chloride Chemistry Skills Chloride Ion Unit Cell One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. The unit cell for sodium chloride is shown in figure 1. Note that there is no lattice. Chloride Ion Unit Cell.
From alchetron.com
Caesium chloride Alchetron, The Free Social Encyclopedia Chloride Ion Unit Cell A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. The unit cell for sodium chloride is shown in figure 1. Based on this we can predict that the chloride ions will. We. Chloride Ion Unit Cell.
From www.gettyimages.com
Sodium Chloride Nacl Molecular Structure HighRes Vector Graphic Chloride Ion Unit Cell The unit cell for sodium chloride is shown in figure 1. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. Based on this we can predict that the chloride ions will. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. The. Chloride Ion Unit Cell.
From www.numerade.com
SOLVED D. SODIUM CHLORIDE UNIT CELL 20. Determine the of sodium Chloride Ion Unit Cell The unit cell for sodium chloride is shown in figure 1. Note that there is no lattice point in the center. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula, nacl. Based on. Chloride Ion Unit Cell.
From www.chegg.com
Solved 2. Look at the unit cell of the CsCl type crystal in Chloride Ion Unit Cell With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula, nacl. Note that there. Chloride Ion Unit Cell.
From utedzz.blogspot.com
Periodic Table Sodium Chloride Periodic Table Timeline Chloride Ion Unit Cell The unit cell for sodium chloride is shown in figure 1. We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. Based. Chloride Ion Unit Cell.
From chemistry-dictionary.yallascience.com
Formula unit of NaCl, sodium chloride Chemistry Dictionary Chloride Ion Unit Cell One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula, nacl. The unit cell for sodium chloride is shown in figure 1. Note that there is no lattice. Chloride Ion Unit Cell.
From chempedia.info
Unit cell of sodium chloride Big Chemical Encyclopedia Chloride Ion Unit Cell Note that there is no lattice point in the center. One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. Based on this we can predict that the chloride ions will. We will look at the ionic structures in the next section, and here focus on the. Chloride Ion Unit Cell.
From stock.adobe.com
Vetor de NaCl structure. Sodium chloride molecule. Salt crystal Chloride Ion Unit Cell A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. The unit cell. Chloride Ion Unit Cell.
From chemistry.stackexchange.com
Unit cell structure of ionic crystal Chemistry Stack Exchange Chloride Ion Unit Cell A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. The unit cell for sodium chloride is shown in figure 1. One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. The unit cell contains four sodium. Chloride Ion Unit Cell.
From chem.libretexts.org
Crystalline Solid Structures Chemistry LibreTexts Chloride Ion Unit Cell We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. Note that there is no lattice point in the center. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. A unit cell is the smallest unit that. Chloride Ion Unit Cell.
From www.britannica.com
Crystal Structure, Lattice, Symmetry Britannica Chloride Ion Unit Cell One cesium ion and one chloride ion are present per unit cell, giving the l:l stoichiometry required by the formula for cesium chloride. We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. A unit cell is the smallest unit that when stacked together repeatedly. Chloride Ion Unit Cell.
From www.chegg.com
Solved Problem 6 (Challenge problem) Cesium chloride Chloride Ion Unit Cell The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula, nacl. Note that there is no lattice point in the center. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. One cesium ion and one chloride ion are present per unit cell, giving. Chloride Ion Unit Cell.
From pressbooks.online.ucf.edu
9.6 Center of Mass University Physics Volume 1 Chloride Ion Unit Cell We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. Note that there is no lattice point in the center. With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. One cesium ion and one chloride ion are. Chloride Ion Unit Cell.
From www.chegg.com
Solved 1A) The unit cell of cesium chloride (CsCl) is Chloride Ion Unit Cell With a radius of 1.60 å, the cesium ion is 88.4% the size of the chloride ion. A unit cell is the smallest unit that when stacked together repeatedly without any gaps can reproduce the entire crystal. The unit cell contains four sodium ions and four chloride ions, giving the 1:1 stoichiometry required by the formula, nacl. We will look. Chloride Ion Unit Cell.