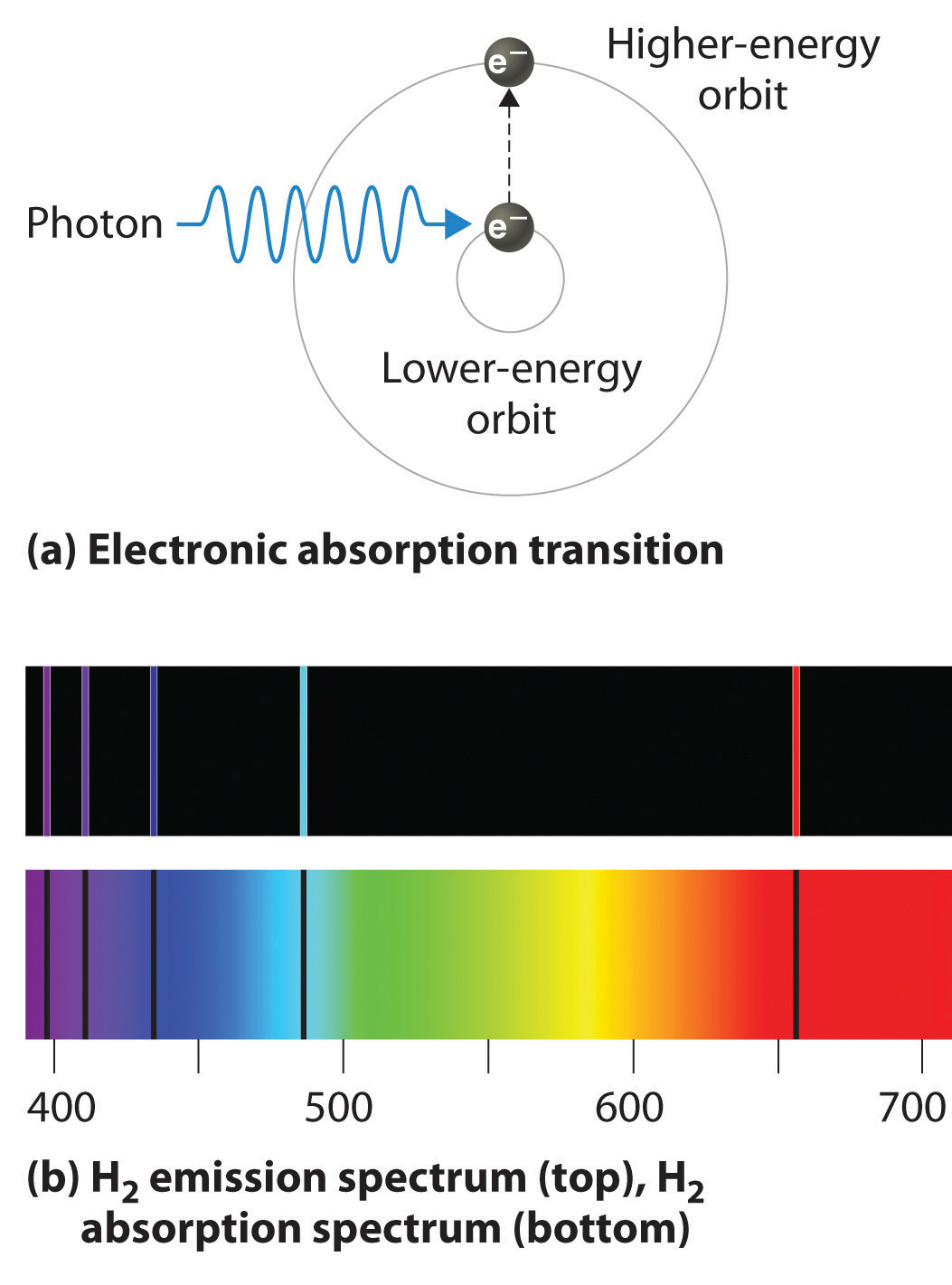
Hydrogen Electronic Transitions . Transition of an electron and spectral lines. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Electron shells and energy levels. Bohr was clever enough to find a way to calculate the electron orbital. The lyman series of lines. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen.
from chem.libretexts.org
The lyman series of lines. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Bohr was clever enough to find a way to calculate the electron orbital. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Electron shells and energy levels. Transition of an electron and spectral lines. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:.
7.3 The Atomic Spectrum of Hydrogen Chemistry LibreTexts
Hydrogen Electronic Transitions In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Electron shells and energy levels. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Bohr was clever enough to find a way to calculate the electron orbital. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. The lyman series of lines. Transition of an electron and spectral lines. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen.
From www.pearson.com
Consider the three electronic transitions in a hydrogen atom show Hydrogen Electronic Transitions The lyman series of lines. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Electron shells and energy levels. Transition of an electron. Hydrogen Electronic Transitions.
From www.youtube.com
CHEMISTRY 101 Electron Transitions in a Hydrogen Atom YouTube Hydrogen Electronic Transitions Electron shells and energy levels. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Bohr was clever enough to find a way to calculate the electron orbital. Transition. Hydrogen Electronic Transitions.
From www.chegg.com
Solved Hydrogen Energy Level DiagramThe orbitals of Hydrogen Electronic Transitions Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Bohr was clever enough to find a way to calculate the electron orbital. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Electron. Hydrogen Electronic Transitions.
From www.youtube.com
Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Hydrogen Electronic Transitions In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Electron shells and energy levels. Transition of an electron and spectral lines. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission. Hydrogen Electronic Transitions.
From mavink.com
Hydrogen Atom Electron Transition Hydrogen Electronic Transitions Transition of an electron and spectral lines. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Bohr was clever enough to find a. Hydrogen Electronic Transitions.
From byjus.com
Hydrogen Spectrum Balmer SeriesDefinitionDiagram Chemistry Byju’s Hydrogen Electronic Transitions Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Transition of an electron and spectral lines. Electron shells and energy levels. Bohr was clever enough to find a. Hydrogen Electronic Transitions.
From chem.libretexts.org
7.3 The Atomic Spectrum of Hydrogen Chemistry LibreTexts Hydrogen Electronic Transitions Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Bohr was clever enough to find a way to calculate the electron orbital. The lyman series of lines. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the. Hydrogen Electronic Transitions.
From www.carolina.com
Hydrogen Spectrum Activity Hydrogen Electronic Transitions Electron shells and energy levels. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy. Hydrogen Electronic Transitions.
From education-portal.com
Electron Transitions & Spectral Lines Video & Lesson Transcript Hydrogen Electronic Transitions Transition of an electron and spectral lines. Bohr was clever enough to find a way to calculate the electron orbital. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. The lyman series of lines. Electron shells and energy levels. Electron transitions the bohr model for an. Hydrogen Electronic Transitions.
From www.researchgate.net
Some electric and dipole transitions in hydrogenlike atom Hydrogen Electronic Transitions Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Bohr was clever enough to find a way to calculate the electron orbital. Electron shells and energy levels. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by. Hydrogen Electronic Transitions.
From byjus.com
Spectral Series Explained along with Hydrogen spectrum, Rydberg formula. Hydrogen Electronic Transitions Bohr was clever enough to find a way to calculate the electron orbital. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. The lyman series of lines. Electron shells and energy levels. Transition of an electron and spectral lines. Figure 7.3.4 electron transitions responsible for the. Hydrogen Electronic Transitions.
From www.chegg.com
Solved Which electron transition in a hydrogen atom is Hydrogen Electronic Transitions Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Electron shells and energy levels. Transition of an electron and spectral lines. The lyman. Hydrogen Electronic Transitions.
From www.slideserve.com
PPT A brief history of atomic structure PowerPoint Presentation, free Hydrogen Electronic Transitions Electron shells and energy levels. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Transition of an electron and spectral lines. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes. Hydrogen Electronic Transitions.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Hydrogen Electronic Transitions Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Electron shells and energy levels. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy. Hydrogen Electronic Transitions.
From www.clutchprep.com
Classify each of the hydrogen atom transitions above. Put le Hydrogen Electronic Transitions Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Bohr was clever enough to find a way to calculate the electron orbital. Electron shells and energy levels. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the. Hydrogen Electronic Transitions.
From www.chegg.com
Solved A Bohrmodel representation of the hydrogen atom is Hydrogen Electronic Transitions Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Electron shells and energy levels. Bohr was clever enough to find a way to calculate the electron orbital. The lyman series of lines. Figure 7.3.4 electron transitions responsible for the various series. Hydrogen Electronic Transitions.
From byjus.com
The energy levels of the hydrogen spectrum is shown in the figure Hydrogen Electronic Transitions Transition of an electron and spectral lines. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Electron transitions the bohr model for an electron transition in hydrogen between. Hydrogen Electronic Transitions.
From nanohub.org
Courses PHYS 342 Modern Physics Public SelfPaced Hydrogen Electronic Transitions Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Transition of an electron and spectral lines. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. In this section we will discuss the. Hydrogen Electronic Transitions.
From stock.adobe.com
Electron transitions for the hydrogen atom Stock Vector Adobe Stock Hydrogen Electronic Transitions In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. The lyman series of lines. Electron shells and energy levels. Bohr was clever enough to find a way to calculate the electron orbital. Transition of an electron and spectral lines. Electron transitions the bohr model for an. Hydrogen Electronic Transitions.
From www.pearson.com
Consider the three electronic transitions in a hydrogen atom show Hydrogen Electronic Transitions Bohr was clever enough to find a way to calculate the electron orbital. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it. Hydrogen Electronic Transitions.
From www.slideserve.com
PPT History of Atomic Theory PowerPoint Presentation, free download Hydrogen Electronic Transitions Transition of an electron and spectral lines. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. The lyman series of lines. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes. Hydrogen Electronic Transitions.
From socratic.org
How much energy is released when "1 mol" of hydrogen atoms transition Hydrogen Electronic Transitions Electron shells and energy levels. Transition of an electron and spectral lines. The lyman series of lines. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n. Hydrogen Electronic Transitions.
From www.chegg.com
Solved When An Electron In A Hydrogen Atom Makes The Tran... Hydrogen Electronic Transitions Electron shells and energy levels. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Transition of an electron and spectral lines. Electron transitions the bohr model for an. Hydrogen Electronic Transitions.
From skippingtheinbetween.blogspot.com
33 The Following Is A Diagram Of Energy States And Transitions In The Hydrogen Electronic Transitions Bohr was clever enough to find a way to calculate the electron orbital. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Transition of an electron and spectral lines. Figure 7.3.4 electron transitions responsible for the various series of lines observed. Hydrogen Electronic Transitions.
From www.chegg.com
Solved Select the electronic transitions for a hydrogen atom Hydrogen Electronic Transitions Electron shells and energy levels. Bohr was clever enough to find a way to calculate the electron orbital. The lyman series of lines. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Transition of an electron and spectral lines. Electron transitions the bohr model for an electron transition in hydrogen between. Hydrogen Electronic Transitions.
From www.youtube.com
Purdue PHYS 342 Modern Physics L6.6 Hydrogen Atom Allowed Hydrogen Electronic Transitions Transition of an electron and spectral lines. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Bohr was clever enough to find a. Hydrogen Electronic Transitions.
From www.naturphilosophie.co.uk
At the Heart of the Hydrogen Atom... NaturPhilosophie Hydrogen Electronic Transitions Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Bohr was clever enough to find a way to calculate the electron orbital. The. Hydrogen Electronic Transitions.
From www.slideserve.com
PPT Chapter 4 Atomic Structure PowerPoint Presentation, free Hydrogen Electronic Transitions Bohr was clever enough to find a way to calculate the electron orbital. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Electron shells and energy levels. In this section we will discuss the energy level of the electron of a. Hydrogen Electronic Transitions.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID398967 Hydrogen Electronic Transitions Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Bohr was clever enough to find a way to. Hydrogen Electronic Transitions.
From www.slideserve.com
PPT Hydrogen Atom PowerPoint Presentation, free download ID4338862 Hydrogen Electronic Transitions Electron shells and energy levels. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Bohr was clever enough to find a way to. Hydrogen Electronic Transitions.
From www.shutterstock.com
Electronic Transitions Hydrogen Spectrum Stock Vector (Royalty Free Hydrogen Electronic Transitions Electron shells and energy levels. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Transition of an electron and spectral lines. Bohr was clever enough to find a way to calculate the electron orbital. The lyman series of lines. Figure 7.3.4. Hydrogen Electronic Transitions.
From pixels.com
Bohr Transitions For Electron Orbitals Photograph by Science Photo Library Hydrogen Electronic Transitions Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. The lyman series of lines. Transition of an electron and spectral lines. Electron shells and energy levels. Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission. Hydrogen Electronic Transitions.
From hydrogenatomgirikosa.blogspot.com
Hydrogen Atom Hydrogen Atom Transition From N=3 To N=2 Hydrogen Electronic Transitions Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. Bohr was clever enough to find a way to calculate the electron orbital. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Transition. Hydrogen Electronic Transitions.
From schematicdiagrampoukes.z13.web.core.windows.net
Electronic Transition Diagram Hydrogen Electronic Transitions Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Electron shells and energy levels. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy. Hydrogen Electronic Transitions.
From www.researchgate.net
Electron transitions for hydrogen. Energy levels are not to scale Hydrogen Electronic Transitions Figure 7.3.4 electron transitions responsible for the various series of lines observed in the emission spectrum of hydrogen. The lyman series of lines. Electron transitions the bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy:. Electron shells and energy levels. Bohr was clever enough. Hydrogen Electronic Transitions.